Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Chemical Foundations: Elements, Atoms, and Ions
Introduction to the Modern Concept of Atomic Structure
Objective
· To understand some important features of subatomic particles.
In the years since Thomson and Rutherford, a great deal has been learned about atomic structure. The simplest view of the atom is that it consists of a tiny nucleus (about cm in diameter) and electrons that move about the nucleus at an average distance of about cm from it (Fig. 4.7). To visualize how small the nucleus is compared with the size of the atom, consider that if the nucleus were the size of a grape, the electrons would be about mile away on average. The nucleus contains protons, which have a positive charge equal in magnitude to the electron’s negative charge, and neutrons, which have almost the same mass as a proton but no charge. The neutrons’ function in the nucleus is not obvious. They may help hold the protons (which repel each other) together to form the nucleus, but we will not be concerned with that here. The relative masses and charges of the electron, proton, and neutron are shown in Table 4.4.
Figure 4.7.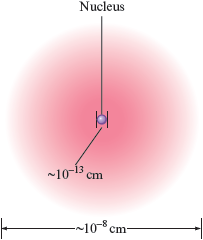
A nuclear atom viewed in cross section. (The symbol means approximately.) This drawing does not show the actual scale. The nucleus is actually much smaller compared with the size of an atom.
Table 4.4. The Mass and Charge of the Electron, Proton, and Neutron
Particle |
Relative Mass* |
Relative Charge |
electron |
||
proton |
||
neutron |
none |
An important question arises at this point: “If all atoms are composed of these same components, why do different atoms have different chemical properties?” The answer lies in the number and arrangement of the electrons. The space in which the electrons move accounts for most of the atomic volume. The electrons are the parts of atoms that “intermingle” when atoms combine to form molecules. Therefore, the number of electrons a given atom possesses greatly affects the way it can interact with other atoms. As a result, atoms of different elements, which have different numbers of electrons, show different chemical behavior. Although the atoms of different elements also differ in their numbers of protons, it is the number of electrons that really determines chemical behavior. We will discuss how this happens in later chapters.