Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Nomenclature
Naming Binary Compounds That Contain a Metal and a Nonmetal (Types I and II)
Objective
· To learn to name binary compounds of a metal and a nonmetal.
As we saw in Section 4.11, when a metal such as sodium combines with a nonmetal such as chlorine, the resulting compound contains ions. The metal loses one or more electrons to become a cation, and the nonmetal gains one or more electrons to form an anion. The resulting substance is called a binary ionic compound . Binary ionic compounds contain a positive ion (cation), which is always written first in the formula, and a negative ion (anion). To name these compounds we simply name the ions.
Chemistry in Focus Sugar of Lead
In ancient Roman society it was common to boil wine in a lead-lined vessel, driving off much of the water to produce a very sweet, viscous syrup called sapa. This syrup was commonly used as a sweetener for many types of food and drink.
We now realize that a major component of this syrup was lead acetate, . This compound has a very sweet taste—hence its original name, sugar of lead.
Many historians believe that the fall of the Roman Empire was due at least in part to lead poisoning, which causes lethargy and mental malfunctions. One major source of this lead was the sapa syrup. In addition, the Romans’ highly advanced plumbing system employed lead water pipes, which allowed lead to be leached into their drinking water.

lucamato/iStockphoto.com
A Roman wine vessel.
Sadly, this story is more relevant to today’s society than you might think. Lead-based solder was widely used for many years to connect the copper pipes in water systems in homes and commercial buildings. There is evidence that dangerous amounts of lead can be leached from these soldered joints into drinking water. In fact, large quantities of lead have been found in the water that some drinking fountains and water coolers dispense. In response to these problems, the U.S. Congress has passed a law banning lead from the solder used in plumbing systems for drinking water.
See Problem 5.1
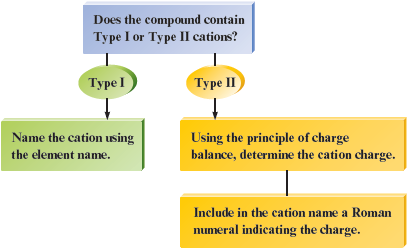
In this section we will consider binary ionic compounds of two types based on the cations they contain. Certain metal atoms form only one cation. For example, the atom always forms , never or . Likewise, always forms , always forms , and always forms . We will call compounds that contain this type of metal atom Type I binary compounds and the cations they contain Type I cations. Examples of Type I cations are , , , and .
Other metal atoms can form two or more cations. For example, can form and and can form and . We will call such ions Type II cations and their compounds Type II binary compounds.
In summary:
Type I compounds: The metal present forms only one type of cation.
Type II compounds: The metal present can form two (or more) cations that have different charges.
Some common cations and anions and their names are listed in Table 5.1. You should memorize these. They are an essential part of your chemical vocabulary.
Table 5.1. Common Simple Cations and Anions
Cation |
Name |
Anion |
Name* |
||
hydrogen |
hydride |
||||
lithium |
fluoride |
||||
sodium |
chloride |
||||
potassium |
bromide |
||||
cesium |
iodide |
||||
beryllium |
oxide |
||||
magnesium |
sulfide |
||||
calcium |
|||||
barium |
|||||
aluminum |
|||||
silver |
|||||
zinc |
Type I Binary Ionic Compounds
The following rules apply for Type I ionic compounds:
Rules for Naming Type I Ionic Compounds
1. The cation is always named first and the anion second.
2. A simple cation (obtained from a single atom) takes its name from the name of the element. For example, is called sodium in the names of compounds containing this ion.
3. A simple anion (obtained from a single atom) is named by taking the first part of the element name (the root) and adding -ide. Thus the ion is called chloride.
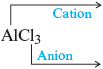
We will illustrate these rules by naming a few compounds. For example, the compound is called sodium iodide. It contains (the sodium cation, named for the parent metal) and (iodide: the root of iodine plus -ide). Similarly, the compound is called calcium oxide because it contains (the calcium cation) and (the oxide anion).
The rules for naming binary compounds are also illustrated by the following examples:
Compound |
Ions Present |
Name |
, |
sodium chloride |
|
, |
potassium iodide |
|
, |
calcium sulfide |
|
, |
cesium bromide |
|
, |
magnesium oxide |
It is important to note that in the formulas of ionic compounds, simple ions are represented by the element symbol: means , means , and so on.
However, when individual ions are shown, the charge is always included. Thus the formula of potassium bromide is written , but when the potassium and bromide ions are shown individually, they are written and .
Interactive Example 5.1. Naming Type I Binary Compounds
Name each binary compound.
a.
b.
c.
Solution
We will name these compounds by systematically following the rules given previously.
· a.
Step 1
Identify the cation and anion. is in Group 1, so we know it will form the ion . Because is in Group 7, it forms the ion .
Step 2
Name the cation. is simply called cesium, the same as the element name.
Step 3
Name the anion. is called fluoride: we use the root name of the element plus -ide.
Step 4
Name the compound by combining the names of the individual ions. The name for is cesium fluoride. (Remember that the name of the cation is always given first.)
· b.
Compound |
Ions Present |
Ion Names |
Comments |
|
|
|
aluminum |
(Group 3) always forms . |
||
chloride |
(Group 7) always forms . |
· The name of is aluminum chloride.
· c.
Compound |
Ions Present |
Ion Names |
Comments |
|
|
|
magnesium |
(Group 2) always forms . |
||
iodide |
(Group 7) gains one electron to form . |
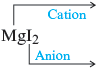
· The name of is magnesium iodide.
Self-Check: Exercise 5.1
· Name the following compounds.
a.
b.
c.
See Problems 5.9 and 5.10.
Example 5.1 reminds us of three things:
1. Compounds formed from metals and nonmetals are ionic.
2. In an ionic compound the cation is always named first.
3. The net charge on an ionic compound is always zero. Thus, in , one of each type of ion ( and ) is required: charge. In , however, three ions are needed to balance the charge of charge. In , two ions are needed for each ion: charge.
Type II Binary Ionic Compounds
So far we have considered binary ionic compounds (Type I) containing metals that always give the same cation. For example, sodium always forms the ion, calcium always forms the ion, and aluminum always forms the ion. As we said in the previous section, we can predict with certainty that each Group 1 metal will give a cation and each Group 2 metal will give a cation. Aluminum always forms .
However, there are many metals that can form more than one type of cation. For example, lead can form or in ionic compounds. Also, iron can produce or , chromium can produce or , gold can produce or , and so on. This means that if we saw the name gold chloride, we wouldn’t know whether it referred to the compound (containing and ) or the compound (containing and three ions). Therefore, we need a way of specifying which cation is present in compounds containing metals that can form more than one type of cation.
Chemists have decided to deal with this situation by using a Roman numeral to specify the charge on the cation. To see how this works, consider the compound . Iron can form or , so we must first decide which of these cations is present. We can determine the charge on the iron cation because we know it must just balance the charge on the two anions (the chloride ions). Thus if we represent the charges as
we know that ? must represent because
The compound , then, contains one ion and two ions. We call this compound iron(II) chloride, where the II tells the charge of the iron cation. That is, is called iron(II). Likewise, is called iron(III). And , which contains one ion and three ions, is called iron(III) chloride. Remember that the Roman numeral tells the charge on the ion, not the number of ions present in the compound.
Note that in the preceding examples the Roman numeral for the cation turned out to be the same as the subscript needed for the anion (to balance the charge). This is often not the case. For example, consider the compound . Because the oxide ion is , for we have
Thus the charge on the lead ion must be to balance the charge of the two oxide ions. The name of is therefore lead(IV) oxide, where the IV indicates the presence of the cation.
There is another system for naming ionic compounds containing metals that form two cations. The ion with the higher charge has a name ending in -ic, and the one with the lower charge has a name ending in -ous. In this system, for example, is called the ferric ion, and is called the ferrous ion. The names for and , in this system, are ferric chloride and ferrous chloride, respectively. Table 5.2 gives both names for many Type II cations. We will use the system of Roman numerals exclusively in this text; the other system is falling into disuse.

© Cengage Learning
Copper(II) sulfate crystals.
Table 5.2. Common Type II Cations
Ion |
Systematic Name |
Older Name |
iron(III) |
ferric |
|
iron(II) |
ferrous |
|
copper(II) |
cupric |
|
copper(I) |
cuprous |
|
cobalt(III) |
cobaltic |
|
cobalt(II) |
cobaltous |
|
tin(IV) |
stannic |
|
tin(II) |
stannous |
|
lead(IV) |
plumbic |
|
lead(II) |
plumbous |
|
mercury(II) |
mercuric |
|
* |
mercury(I) |
mercurous |
To help distinguish between Type I and Type II cations, remember that Group 1 and 2 metals are always Type I. On the other hand, transition metals are almost always Type II.
Rules for Naming Type II Ionic Compounds
1. The cation is always named first and the anion second.
2. Because the cation can assume more than one charge, the charge is specified by a Roman numeral in parentheses.
Interactive Example 5.2. Naming Type II Binary Compounds
Give the systematic name of each of the following compounds.
a.
b.
c.
d.
e.
Solution
All these compounds include a metal that can form more than one type of cation; thus we must first determine the charge on each cation. We do this by recognizing that a compound must be electrically neutral; that is, the positive and negative charges must balance exactly. We will use the known charge on the anion to determine the charge of the cation.
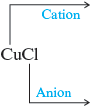
a. In we recognize the anion as . To determine the charge on the copper cation, we invoke the principle of charge balance.
In this case, must be because . Thus the copper cation must be . Now we can name the compound by using the regular steps.
Compound |
Ions Present |
Ion Names |
Comments |
||
|
|
copper(I) |
Copper forms other cations (it is a transition metal), so we must include the to specify its charge. |
|||
chloride |
|||||
The name of is copper(I) chloride.
b. In we recognize the anion. To yield zero net charge, the cation must be .
Compound |
Ions Present |
Ion Names |
Comments |
|
|
|
mercury(II) |
The II is necessary to specify the charge. |
||
oxide |
c. The name of is mercury(II) oxide.
d. Because contains three anions, the charge on the iron cation must be .
Compound |
Ions Present |
Ion Names |
Comments |
||
|
|
iron(III) |
Iron is a transition metal and requires a III to specify the charge on the cation. |
|||
oxide |
|||||
e. The name of is iron(III) oxide.
f. contains two anions, so the charge on the manganese cation is .
Compound |
Ions Present |
Ion Names |
Comments |
||
|
|
manganese(IV) |
Manganese is a transition metal and requires a IV to specify the charge on the cation. |
|||
oxide |
|||||
g. The name of is manganese(IV) oxide.
h. Critical Thinking
o We can use the periodic table to tell us something about the stable ions formed by many atoms. For example, the atoms in column 1 always form ions. The transition metals, however, can form more than one type of stable ion. What if each transition metal ion had only one possible charge? How would the naming of compounds be different?
i. Because contains four anions, the charge on the lead cation is .
Compound |
Ions Present |
Ion Names |
Comments |
|
|
|
lead(IV) |
Lead forms both and , so a Roman numeral is required. |
||
chloride |
j. The name for is lead(IV) chloride.
The use of a Roman numeral in a systematic name for a compound is required only in cases where more than one ionic compound forms between a given pair of elements. This occurs most often for compounds that contain transition metals, which frequently form more than one cation. Metals that form only one cation do not need to be identified by a Roman numeral. Common metals that do not require Roman numerals are the Group 1 elements, which form only ions; the Group 2 elements, which form only ions; and such Group 3 metals as aluminum and gallium, which form only ions.
As shown in Example 5.2, when a metal ion that forms more than one type of cation is present, the charge on the metal ion must be determined by balancing the positive and negative charges of the compound. To do this, you must be able to recognize the common anions and you must know their charges (see Table 5.1).
Interactive Example 5.3. Naming Binary Ionic Compounds: Summary
Give the systematic name of each of the following compounds.
a.
b.
c.
d.
Solution
a.
Compound |
Ions and Names |
Compound Name |
Comments |
|
|
cobalt(II) |
cobalt(II) bromide |
Cobalt is a transition metal; the name of the compound must have a Roman numeral. The two ions must be balanced by a cation. |
bromide |
b.
Compound |
Ions and Names |
Compound Name |
Comments |
||
|
|
calcium |
calcium chloride |
Calcium, a Group 2 metal, forms only the ion. A Roman numeral is not necessary. |
||
chloride |
|||||
c.
Compound |
Ions and Names |
Compound Name |
Comments |
||
|
|
aluminum |
aluminum oxide |
Aluminum forms only . A Roman numeral is not necessary. |
||
oxide |
|||||
d.
Compound |
Ions and Names |
Compound Name |
Comments |
||
|
|
chromium(III) |
chromium(III) chloride |
Chromium is a transition metal. The name of the com pound must have a Roman numeral. contains . |
||
chloride |
|||||
Self-Check: Exercise 5.2
· Give the names of the following compounds.
a. and
b. and
c.
d.
e.
See Problems 5.9, 5.10, and 5.13, 5.14, 5.15, and 5.16.
The following flowchart is useful when you are naming binary ionic compounds: