Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Nomenclature
Naming Binary Compounds: A Review
Objective
· To review the naming of Type I, Type II, and Type III binary compounds.
Because different rules apply for naming various types of binary compounds, we will now consider an overall strategy to use for these compounds. We have considered three types of binary compounds, and naming each of them requires different procedures.
Type I: Ionic compounds with metals that always form a cation with the same charge
Type II: Ionic compounds with metals (usually transition metals) that form cations with various charges
Type III: Compounds that contain only nonmetals
In trying to determine which type of compound you are naming, use the periodic table to help identify metals and nonmetals and to determine which elements are transition metals.
The flowchart given in Fig. 5.1 should help you as you name binary compounds of the various types.
Figure 5.1.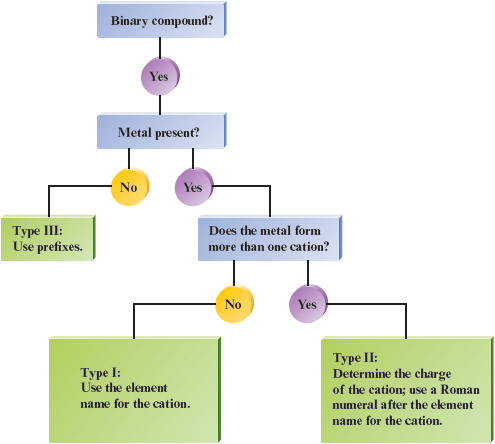
A flowchart for naming binary compounds.
Interactive Example 5.6. Naming Binary Compounds: Summary
Name the following binary compounds.
a.
b.
c.
d.
e.
f.
g.
Solution
a. 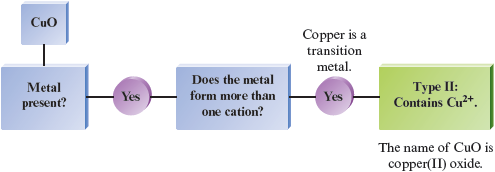
b. 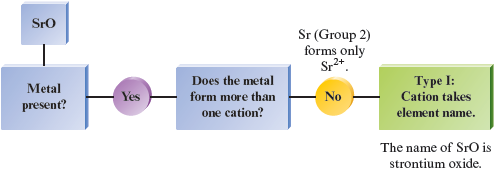
c. 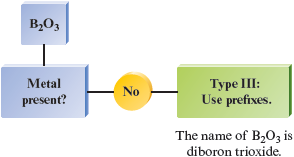
d. 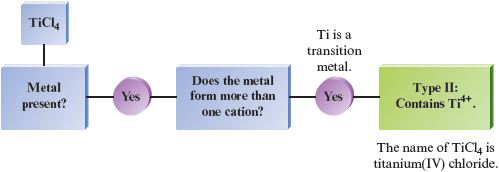
e. 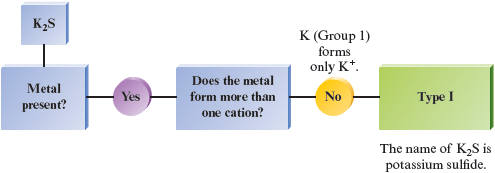
f. 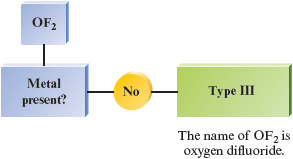
g. 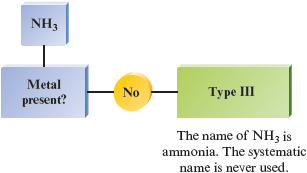
Self-Check: Exercise 5.5
· Name the following binary compounds.
a.
b.
c.
d.
e.
f.
See Problems 5.19, 5.20, 5.21, and 5.22.
Chemistry in Focus Chemophilately
Philately is the study of postage stamps. Chemophilately, a term coined by the Israeli chemist Zvi Rappoport, refers to the study of stamps that have some sort of chemical connection. Collectors estimate that more than chemical-related stamps have been printed throughout the world. Relatively few of these stamps have been produced in the United States. One example is a stamp honoring minerals that shows a copper nugget.
Chemists also have been honored on U.S. postage stamps. One example is a stamp printed in 1993 honoring Percy L. Julian, an African-American chemist who was the grandson of slaves. Julian is noted for his synthesis of steroids used to treat glaucoma and rheumatoid arthritis. As a holder of more than 100 patents, he was inducted into the National Inventors Hall of Fame in 1990.
In 1983 the United States issued a stamp honoring Joseph Priestley, whose experiments led to the discovery of oxygen.
A Russian stamp from 2009 pictures Dmitri Mendeleev, who, in 1869, arranged the known elements in the present form of the periodic table. Mendeleev’s arrangement allowed for the prediction of yet unknown elements and their properties.
In 2008, a stamp was issued that honors Linus C. Pauling, who pioneered the concept of the chemical bond. Pauling received two Nobel Prizes: one for his work on chemical bonds and the other for his work championing world peace. His stamp includes drawings of red blood cells to commemorate his work on the study of hemoglobin, which led to the classification of sickle cell anemia as a molecular disease.
Marie Curie also received two Nobel Prizes and was the first person to be so honored. She shared her Nobel Prize in Physics in 1903 with her husband, Pierre Curie, and Henri Becquerel for their research on radiation. She was the sole winner of the Nobel Prize in Chemistry in 1911 for the discovery and study of the elements radium and polonium. In 2011 (The International Year of Chemistry), many countries issued stamps honoring the 100th anniversary of Marie Curie winning the Nobel Prize in Chemistry.
Postal chemistry also shows up in postmarks from places in the United States with chemical names. Examples include Radium, ; Neon, ; Boron, ; Bromide, ; and Telluride, .
Chemophilately—further proof that chemistry is everywhere!