Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Reactions in Aqueous Solutions
Other Ways to Classify Reactions
Objective
· To consider additional classes of chemical reactions.
So far in this chapter we have classified chemical reactions in several ways. The most commonly used of these classifications are
· Precipitation reactions
· Acid—base reactions
· Oxidation—reduction reactions
However, there are still other ways to classify reactions that you may encounter in your future studies of chemistry. We will consider several of these in this section.
Combustion Reactions
Many chemical reactions that involve oxygen produce energy (heat) so rapidly that a flame results. Such reactions are called combustion reactions . We have considered some of these reactions previously. For example, the methane in natural gas reacts with oxygen according to the following balanced equation:
This reaction produces the flame of the common laboratory burner and is used to heat most homes in the United States. Recall that we originally classified this reaction as an oxidation—reduction reaction in Section 7.5. Thus we can say that the reaction of methane with oxygen is both an oxidation—reduction reaction and a combustion reaction. Combustion reactions, in fact, are a special class of oxidation—reduction reactions (Fig. 7.11).
Figure 7.11.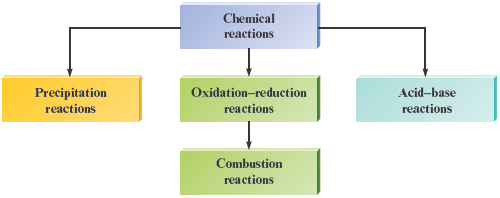
Classes of reactions. Combustion reactions are a special type of oxidation—reduction reaction.
There are many combustion reactions, most of which are used to provide heat or electricity for homes or businesses or energy for transportation. Some examples are:
· Combustion of propane (used to heat some rural homes)
· Combustion of gasoline* (used to power cars and trucks)
· Combustion of coal* (used to generate electricity)
Synthesis (Combination) Reactions
One of the most important activities in chemistry is the synthesis of new compounds. Each of our lives has been greatly affected by synthetic compounds such as plastic, polyester, and aspirin. When a given compound is formed from simpler materials, we call this a synthesis (or combination) reaction .
In many cases synthesis reactions start with elements, as shown by the following examples:
● |
Synthesis of water |
|
● |
Synthesis of carbon dioxide |
|
● |
Synthesis of nitrogen monoxide |
Notice that each of these reactions involves oxygen, so each can be classified as an oxidation—reduction reaction. The first two reactions are also commonly called combustion reactions because they produce flames. The reaction of hydrogen with oxygen to produce water, then, can be classified three ways: as an oxidation—reduction reaction, as a combustion reaction, and as a synthesis reaction.
There are also many synthesis reactions that do not involve oxygen:
● |
Synthesis of sodium chloride |
|
● |
Synthesis of magnesium fluoride
|
We have discussed the formation of sodium chloride before and have noted that it is an oxidation—reduction reaction; uncharged sodium atoms lose electrons to form ions, and uncharged chlorine atoms gain electrons to form ions. The synthesis of magnesium fluoride is also an oxidation—reduction reaction because and ions are produced from the uncharged atoms.
We have seen that synthesis reactions in which the reactants are elements are oxidation—reduction reactions as well. In fact, we can think of these synthesis reactions as another subclass of the oxidation—reduction class of reactions.
Decomposition Reactions
In many cases a compound can be broken down into simpler compounds or all the way to the component elements. This is usually accomplished by heating or by the application of an electric current. Such reactions are called decomposition reactions . We have discussed decomposition reactions before, including
· Decomposition of water
· Decomposition of mercury(II) oxide
Because is involved in the first reaction, we recognize it as an oxidation—reduction reaction. In the second reaction, , which contains and ions, is decomposed to the elements, which contain uncharged atoms. In this process each gains two electrons and each loses two electrons, so this is both a decomposition reaction and an oxidation—reduction reaction.
A decomposition reaction, in which a compound is broken down into its elements, is just the opposite of the synthesis (combination) reaction, in which elements combine to form the compound. For example, we have just discussed the synthesis of sodium chloride from its elements. Sodium chloride can be decomposed into its elements by melting it and passing an electric current through it:
There are other schemes for classifying reactions that we have not considered. However, we have covered many of the classifications that are commonly used by chemists as they pursue their science in laboratories and industrial plants.
It should be apparent that many important reactions can be classified as oxidation—reduction reactions. As shown in Fig. 7.12, various types of reactions can be viewed as subclasses of the overall oxidation—reduction category.
Figure 7.12.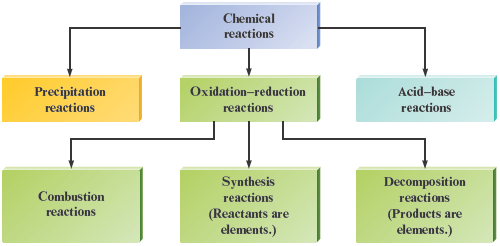
Summary of classes of reactions.
Critical Thinking
· Dalton believed that atoms were indivisible. Thomson and Rutherford helped to show that this was not true. What if atoms were indivisible? How would this affect the types of reactions you have learned about in this chapter?
Interactive Example 7.6. Classifying Reactions
Classify each of the following reactions in as many ways as possible.
a.
b.
c.
d.
e.
f.
Solution
a. This is both a synthesis reaction (elements combine to form a compound) and an oxidation—reduction reaction (uncharged potassium and chlorine atoms are changed to and ions in ).
b. This is an oxidation—reduction reaction. Iron is present in as ions and in elemental iron, , as uncharged atoms. So each must gain three electrons to form . The reverse happens to aluminum, which is present initially as uncharged aluminum atoms, each of which loses three electrons to give ions in . Note that this reaction might also be called a single-replacement reaction because is switched from to .
c. This is both a synthesis reaction (elements combine to form a compound) and an oxidation—reduction reaction (each magnesium atom loses two electrons to give ions in , and each oxygen atom gains two electrons to give in ).
d. This is an acid—base reaction. It might also be called a double-displacement reaction because and “switch partners.”
e. This is a precipitation reaction that might also be called a double-displacement reaction in which the anions and are exchanged.
f. This is a decomposition reaction (a compound breaks down into elements). It also is an oxidation—reduction reaction, because the ions in ( and ) are changed to uncharged atoms in the elements and . That is, electrons are transferred from to in the reaction.
Self-Check: Exercise 7.4
· Classify each of the following reactions in as many ways as possible.
a.
b.
c.
d.
e.
f.
g.
h.
See Problems 7.53 and 7.54.