Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Energy
Temperature and Heat
Objective
· To understand the concepts of temperature and heat.
What does the temperature of a substance tell us about that substance? Put another way, how is warm water different from cold water? The answer lies in the motions of the water molecules. Temperature is a measure of the random motions of the components of a substance. That is, the molecules in warm water are moving around more rapidly than the molecules in cold water.
Consider an experiment in which we place kg of hot water next to kg of cold water in an insulated box. The water samples are separated from each other by a thin metal plate (Fig. 10.2). You already know what will happen: the hot water will cool down and the cold water will warm up.
Figure 10.2.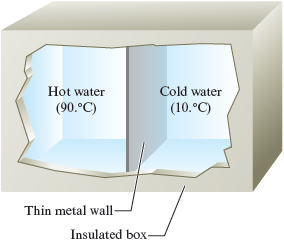
Equal masses of hot water and cold water separated by a thin metal wall in an insulated box.
Assuming that no energy is lost to the air, can we determine the final temperature of the two samples of water? Let’s consider how to think about this problem.
First picture what is happening. Remember that the molecules in the hot water are moving faster than those in the cold water (Fig. 10.3). As a result, energy will be transferred through the metal wall from the hot water to the cold water. This energy transfer will cause the molecules in the hot water to slow down and the molecules in the cold water to speed up.
Figure 10.3.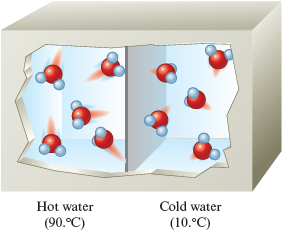
The molecules in hot water have much greater random motions than the molecules in cold water.
Thus we have a transfer of energy from the hot water to the cold water. This flow of energy is called heat. Heat can be defined as a flow of energy due to a temperature difference. What will eventually happen? The two water samples will reach the same temperature (Fig. 10.4). At this point, how does the energy lost by the hot water compare to the energy gained by the cold water? They must be the same (remember that energy is conserved).
Figure 10.4.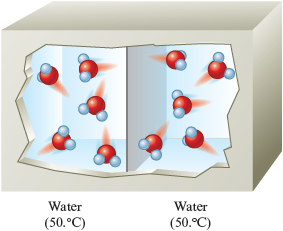
The water samples now have the same temperature and have the same random motions.
We conclude that the final temperature is the average of the original temperatures:
For the hot water, the temperature change is
The temperature change for the cold water is
In this example, the masses of hot water and cold water are equal. If they were unequal, this problem would be more complicated.
Let’s summarize the ideas we have introduced in this section. Temperature is a measure of the random motions of the components of an object. Heat is a flow of energy due to a temperature difference. We say that the random motions of the components of an object constitute the thermal energy of that object. The flow of energy called heat is the way in which thermal energy is transferred from a hot object to a colder object.