Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Energy
Energy and Our World
Objective
· To consider the energy resources of our world.
Woody plants, coal, petroleum, and natural gas provide a vast resource of energy that originally came from the sun. By the process of photosynthesis, plants store energy that can be claimed by burning the plants themselves or the decay products that have been converted over millions of years to fossil fuels . Although the United States currently depends heavily on petroleum for energy, this dependency is a relatively recent phenomenon, as shown in Fig. 10.7. In this section we discuss some sources of energy and their effects on the environment.
Figure 10.7.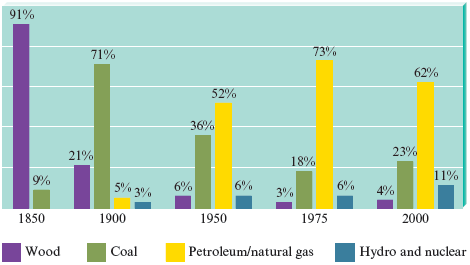
Energy sources used in the United States.
Petroleum and Natural Gas
Although how they were produced is not completely understood, petroleum and natural gas were most likely formed from the remains of marine organisms that lived approximately 500 million years ago. Because of the way these substances were formed they are called fossil fuels. Petroleum is a thick, dark liquid composed mostly of compounds called hydrocarbons that contain carbon and hydrogen. (Carbon is unique among elements in the extent to which it can bond to itself to form chains of various lengths.) Table 10.2 gives the formulas and names for several common hydrocarbons. Natural gas , usually associated with petroleum deposits, consists mostly of methane, but it also contains significant amounts of ethane, propane, and butane.
Table 10.2. Names and Formulas for Some Common Hydrocarbons
Formula |
Name |
Methane |
|
Ethane |
|
Propane |
|
Butane |
|
Pentane |
|
Hexane |
|
Heptane |
|
Octane |
The composition of petroleum varies somewhat, but it includes mostly hydrocarbons having chains that contain from to more than carbons. To be used efficiently, the petroleum must be separated into fractions by boiling. The lighter molecules (having the lowest boiling points) can be boiled off, leaving the heavier ones behind. The commercial uses of various petroleum fractions are shown in Table 10.3.
Table 10.3. Uses of the Various Petroleum Fractions
Petroleum Fraction in Terms of Numbers of Carbon Atoms |
Major Uses |
Gasoline |
|
Kerosene Jet fuel |
|
Diesel fuel Heating oil Lubricating oil |
|
Asphalt |
At the end of the twentieth century there was increasing concern that the global supplies of fossil fuels were being rapidly depleted. However, a new technology called hydraulic fracturing or fracking has changed the situation significantly. Fracking involves injecting a mixture of water, sand, and chemicals at high pressures through a well drilled deep into rock layers. The high pressures involved cause the rock layer to fracture, allowing deep pockets of petroleum and natural gas to escape and be recovered. This technique has the potential to produce huge amounts of previously unavailable petroleum. For example, it is estimated that recoverable supplies of natural gas in deep shale deposits amount to over trillion cubic meters of natural gas. Thus the introduction of fracking has completely altered the thinking about future energy sources.
The petroleum era began when the demand for lamp oil during the Industrial Revolution outstripped the traditional sources: animal fats and whale oil. In response to this increased demand, Edwin Drake drilled the first oil well in 1859 at Titusville, Pennsylvania. The petroleum from this well was refined to produce kerosene (fraction ), which served as an excellent lamp oil. Gasoline (fraction ) had limited use and was often discarded. This situation soon changed. The development of the electric light decreased the need for kerosene, and the advent of the “horseless carriage” with its gasoline-powered engine signaled the birth of the gasoline age.
As gasoline became more important, new ways were sought to increase the yield of gasoline obtained from each barrel of petroleum. William Burton invented a process at Standard Oil of Indiana called pyrolytic (high-temperature) cracking. In this process, the heavier molecules of the kerosene fraction are heated to about , causing them to break (crack) into the smaller molecules of hydrocarbons in the gasoline fraction. As cars became larger, more efficient internal combustion engines were designed. Because of the uneven burning of the gasoline then available, these engines “knocked,” producing unwanted noise and even engine damage. Intensive research to find additives that would promote smoother burning produced tetraethyl lead, , a very effective “antiknock” agent.
The addition of tetraethyl lead to gasoline became a common practice, and by 1960, gasoline contained as much as g of lead per gallon. As we have discovered so often in recent years, technological advances can produce environmental problems. To prevent air pollution from automobile exhaust, catalytic converters have been added to car exhaust systems. The effectiveness of these converters, however, is destroyed by lead. The use of leaded gasoline also greatly increased the amount of lead in the environment, where it can be ingested by animals and humans. For these reasons, the use of lead in gasoline has been phased out, requiring extensive (and expensive) modifications of engines and of the gasoline refining process.
Coal
Coal was formed from the remains of plants that were buried and subjected to high pressure and heat over long periods of time. Plant materials have a high content of cellulose, a complex molecule whose empirical formula is but whose molar mass is approximately g/mol. After the plants and trees that grew on the earth at various times and places died and were buried, chemical changes gradually lowered the oxygen and hydrogen content of the cellulose molecules. Coal “matures” through four stages: lignite, subbituminous, bituminous, and anthracite. Each stage has a higher carbon-to-oxygen and carbon-to-hydrogen ratio; that is, the relative carbon content gradually increases. Typical elemental compositions of the various coals are given in Table 10.4. The energy available from the combustion of a given mass of coal increases as the carbon content increases. Anthracite is the most valuable coal, and lignite is the least valuable.

cbpix/iStockphoto.com
Table 10.4. Element Composition of Various Types of Coal
|
Mass Percent of Each Element |
|||||
Type of Coal |
|||||
Lignite |
|||||
Subbituminous |
|||||
Bituminous |
|||||
Anthracite |
|||||
Coal is an important and plentiful fuel in the United States, currently furnishing approximately of our energy. As the supply of petroleum decreases, the share of the energy supply from coal could eventually increase to as high as . However, coal is expensive and dangerous to mine underground, and the strip mining of fertile farmland in the Midwest or of scenic land in the West causes obvious problems. In addition, the burning of coal, especially high-sulfur coal, yields air pollutants such as sulfur dioxide, which, in turn, can lead to acid rain. However, even if coal were pure carbon, the carbon dioxide produced when it was burned would still have significant effects on the earth’s climate.
Effects of Carbon Dioxide on Climate
The earth receives a tremendous quantity of radiant energy from the sun, about of which is reflected back into space by the earth’s atmosphere. The remaining energy passes through the atmosphere to the earth’s surface. Some of this energy is absorbed by plants for photosynthesis and some by the oceans to evaporate water, but most of it is absorbed by soil, rocks, and water, increasing the temperature of the earth’s surface. This energy is, in turn, radiated from the heated surface mainly as infrared radiation, often called heat radiation.
The atmosphere, like window glass, is transparent to visible light but does not allow all the infrared radiation to pass back into space. Molecules in the atmosphere, principally and , strongly absorb infrared radiation and radiate it back toward the earth, as shown in Fig. 10.8. A net amount of thermal energy is retained by the earth’s atmosphere, causing the earth to be much warmer than it would be without its atmosphere. In a way, the atmosphere acts like the glass of a greenhouse, which is transparent to visible light but absorbs infrared radiation, thus raising the temperature inside the building. This greenhouse effect is seen even more spectacularly on Venus, where the dense atmosphere is thought to be responsible for the high surface temperature of that planet.
Figure 10.8.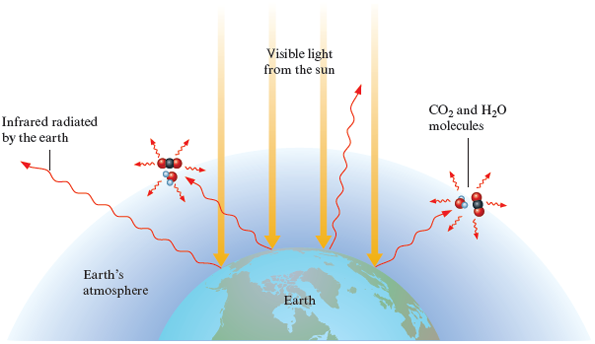
The earth’s atmosphere is transparent to visible light from the sun. This visible light strikes the earth, and part of it is changed to infrared radiation. The infrared radiation from the earth’s surface is strongly absorbed by , , and other molecules present in smaller amounts (for example, and ) in the atmosphere. In effect, the atmosphere traps some of the energy, acting like the glass in a greenhouse and keeping the earth warmer than it would otherwise be.
Thus the temperature of the earth’s surface is controlled to a significant extent by the carbon dioxide and water content of the atmosphere. The effect of atmospheric moisture (humidity) is readily apparent in the Midwest, for example. In summer, when the humidity is high, the heat of the sun is retained well into the night, giving very high nighttime temperatures. In winter, the coldest temperatures always occur on clear nights, when the low humidity allows efficient radiation of energy back into space.
Chemistry in Focus Seeing the Light
We are about to have a revolution in lighting. The incandescent light bulb developed by Thomas Edison in the late nineteenth century still dominates our lighting systems. However, this is about to change because Edison’s light bulb is so inefficient: about of the energy goes to heat instead of light. In the United States, of total electricity production goes for lighting, for a cost of about million. Globally, illumination consumes about of electricity, and demand for lighting is expected to grow by in the next years. Given energy prices and the problems associated with global warming, we must find more efficient lighting devices.
In the short term, the answer appears to be compact fluorescent lights (CFLs). These bulbs, which have a screw-type base, draw only about as much energy as incandescent bulbs for a comparable amount of light production. Although they cost four times as much, CFLs last ten times as long as incandescent bulbs. CFLs produce light from a type of compound called a phosphor that coats the inner walls of the bulb. The phosphor is mixed with a small amount of mercury (about mg per bulb). When the bulb is turned on, a beam of electrons is produced. The electrons are absorbed by mercury atoms, which are caused to emit ultraviolet (UV) light. This UV light is absorbed by phosphor, which then emits visible light (a process called fluorescence). It is estimated that replacing all of the incandescent bulbs in our homes with CFLs would reduce our electrical demand in the United States by the equivalent of the power produced by new -MW nuclear power plants. This is a very significant savings.
Although the amount of mercury in each bulb is small (breaking a single CFL would not endanger a normal adult), recycling large numbers of CFLs does present potential pollution hazards. Research is now under way to find ways to alleviate this danger. For example, Professor Robert Hurt and his colleagues at Brown University have found that selenium prepared as tiny particles has a very high affinity for mercury and can be used in recycling operations to prevent dangerous occupational exposure to mercury.
Another type of lighting device that is now economical enough to be used widely is the light-emitting diode (LED). An LED is a solid-state semiconductor designed to emit visible light when its electrons fall to lower energy levels. The tiny glowing light that indicates an audio system or television is on is an LED. In recent years, LEDs have been used in traffic lights, turn signals on cars, flashlights, and street lights. The use of LEDs for holiday lighting is rapidly increasing. It is estimated that LEDs eventually will reduce energy consumption for holiday lighting by . The light production of LEDs per amount of energy consumed has increased dramatically in recent months, and the costs are decreasing steadily. Although LED lights are more expensive than CFLs, they last more than years. Thus dramatic changes are occurring in the methods for lighting, and we all need to do our part to make our lives more energy efficient.
The atmosphere’s water content is controlled by the water cycle (evaporation and precipitation), and the average has remained constant over the years. However, as fossil fuels have been used more extensively, the carbon dioxide concentration has increased—up about from 1880 to the present. Projections indicate that the carbon dioxide content of the atmosphere may be double in the twenty-first century what it was in 1880. This trend could increase the earth’s average temperature by as much as , causing dramatic changes in climate and greatly affecting the growth of food crops.
How well can we predict the long-term effects of carbon dioxide? Because weather has been studied for a period of time that is minuscule compared with the age of the earth, the factors that control the earth’s climate in the long range are not clearly understood. For example, we do not understand what causes the earth’s periodic ice ages. So it is difficult to estimate the effects of the increasing carbon dioxide levels.
In fact, the variation in the earth’s average temperature over the past century is somewhat confusing. In the northern latitudes during the past century, the average temperature rose by over a period of years, then cooled by during the next years, and finally warmed by in the succeeding years. Such fluctuations do not match the steady increase in carbon dioxide. However, in southern latitudes and near the equator during the past century, the average temperature showed a steady rise totaling . This figure is in reasonable agreement with the predicted effect of the increasing carbon dioxide concentration over that period. Another significant fact is that the last years of the twentieth century have been the warmest decade on record.
Although the exact relationship between the carbon dioxide concentration in the atmosphere and the earth’s temperature is not known at present, one thing is clear: The increase in the atmospheric concentration of carbon dioxide is quite dramatic (Fig. 10.9). We must consider the implications of this increase as we consider our future energy needs.
Figure 10.9.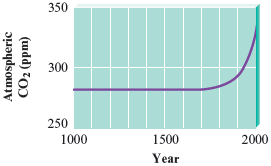
The atmospheric concentration over the past years, based on ice core data and direct readings (since 1958). Note the dramatic increase in the past years.
New Energy Sources
As we search for the energy sources of the future, we need to consider economic, climatic, and supply factors. There are several potential energy sources: the sun (solar), nuclear processes (fission and fusion), biomass (plants), and synthetic fuels. Direct use of the sun’s radiant energy to heat our homes and run our factories and transportation systems seems a sensible long-term goal. But what do we do now? Conservation of fossil fuels is one obvious step, but substitutes for fossil fuels also must be found. There is much research going on now to solve this problem.
Critical Thinking
· A government study concludes that burning fossil fuels to power our automobiles causes too much pollution. What if Congress decided that all cars and trucks must be powered by batteries? Would this solve the air pollution problems caused by transportation?