Liquid-State Physical Chemistry: Fundamentals, Modeling, and Applications (2013)
4. Describing Liquids: Phenomenological Behavior
4.3. Corresponding States
The CP is characterized by (∂P/∂V)T = (∂2P/∂V2)T = 0, and this leads for the vdW equation to (with V the molar volume Vm)
(4.7) ![]()
Defining the reduced quantities
(4.8) ![]()
we can write the EoS in a nondimensional form, usually called the reduced vdW equation of state, and given by
(4.9) ![]()
As this reduced equation contains no constants characteristic of a particular fluid, the result is (theoretically) valid for all fluids, and represents an example of the principle of corresponding states (PoCS). Quite apart from the applicability of the reduced vdW EoS, the PoCS is an experimental fact which asserts that, for a group of similar substances, the EoS can be written in the form
(4.10) ![]()
where Ω is the same function for all the substances of the group.
In order to estimate a and b, the values Pcri and Tcri (see Appendix E) can be used. Another way of determining a and b is to fit P to the vdW equation for a range of temperatures. It will be clear that different procedures yield (slightly) different values. The (approximate) validity of the PoCS is indicated by the fact that the compression factor at critical conditions Zcri = PcriVcri/RTcri is approximately constant for a set of spherical, nonpolar molecules at (0.293 ± 0.005), where ± indicates the sample standard deviation (Table 4.3). Note that for the vdW fluid Zcri = 3/8 = 0.375. Perhaps more convincing is a plot of Z versus Pred, as given in Figure 4.4 for several molecules, which shows that the curves for a certain, fixed Tred nicely coincide.
Table 4.3 Critical reduced compression factor and surface tension for several simple molecules.
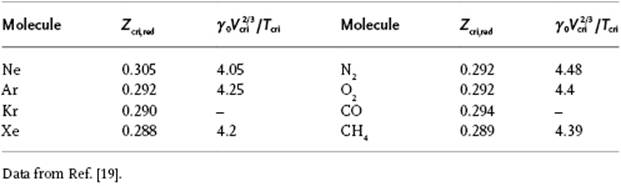
Figure 4.4 Compression factor Z as a function of the reduced pressure for various fluids, illustrating the principle of corresponding states. Data from Ref. [17].
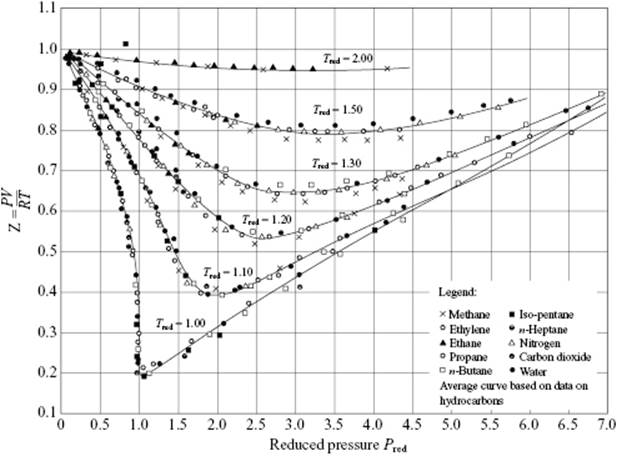
Another example of the PoCS is shown in Figure 4.5, where the second virial coefficient B/Vcri versus T/Tcri for Ar, Kr, Xe and CH4 is shown. The curve can be described well by the expression as derived for the square-well potential (see Chapter 3) with a hard-core diameter σ, a depth ε and a range 1.5σ, and is given by
(4.11) ![]()
Figure 4.5 (a) The common curve of T/Tcri versus ρ/ρcri for various fluids; (b) The common curve of B/Vcri versus T/Tcri for Ar, Kr, Xe, and CH4. Both curves represent examples of the PoCS.
The parameters σ and ε are given by ⅔πNAσ3 = 0.447Vcri and ε = 0.936kTcri. As a final example, Figure 4.5 also shows ρ/ρcri versus T/Tcri. The curve is well fitted by
(4.12) ![]()
where ρL and ρG represent the densities of the liquid and gas, respectively. The first of these expressions represents the law of rectilinear diameters, which states that the average density of gas and liquid is a linear function of its temperature.
The PoCS implies that the intermolecular potential has a similar shape for the molecules within the class of molecules considered. For a two-parameter intermolecular potential of the form ϕ(r) = εf(r/σ), the distances are scaled by σ, while the energy is scaled by ε. Consequently, we can scale the pressure P according to P* = σ3P/ε, the volume V according to V* = V/σ3, and the temperature T according to T* = kT/ε. Finally, the configurational partition function (see Chapter 5) scales according to Q* = Q/σ3N. In this way, the pressure becomes
(4.13) ![]()
In this dimensionless representation, the P–V curves of a set of molecules should coincide for the same T*, as illustrated in Figure 4.4 using Tred.
4.3.1 Extended Principle*
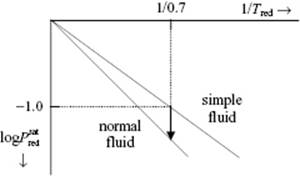
The conventional corresponding state approach deals with two parameters, for example, using the compression factor Z = Z(Tred,Pred). For simple, (more or less) spherical molecules this approach works reasonably well, as evidenced by the “universal” value of the compression factor Z [3]. In fact, any EoS that contains only two parameters can be mathematically reduced likewise. However, the reduction is essentially based on dimensional analysis with force, length and temperature as basic dimensions. In the case of categories of molecules for which extra dimensions are important, one does not expect the principle to apply. A significant improvement in describing experimental results can be obtained by extending the concept to three parameters, of which the best known approaches are due to Riedel [4] and Pitzer [5]. Both approaches use the pressure P versus temperature T. Riedel used the parameter α = (d lnP/d lnT)T=Tcri taken along the saturation curve, while Pitzer et al. used a parameter that can be obtained if we plot the logarithm of the saturated vapor pressure in reduced units versus 1/Tred. In this representation, approximately straight lines are obtained, that is, ![]() . The two-parameter principle predicts that the slope S is the same for all substances. If we plot the vapor pressures of simple fluids (spherical molecules such as Ar, CH4, …), they indeed all fall more or less on the same line that appears to go through
. The two-parameter principle predicts that the slope S is the same for all substances. If we plot the vapor pressures of simple fluids (spherical molecules such as Ar, CH4, …), they indeed all fall more or less on the same line that appears to go through ![]() at Tred = 0.7. However, other, less-spherical molecules do have different slopes. These facts are used to define the so-called acentric factor
at Tred = 0.7. However, other, less-spherical molecules do have different slopes. These facts are used to define the so-called acentric factor ![]() (Figure 4.6). This apparently arbitrary definition was chosen because of the ease and precision with which this quantity can be determined (contrary to Riedel's α, which might be difficult to determine precisely). Since at T/Tcri = 0.7 the vapor pressure of simple fluids is close to 0.1 Pcri, the acentric factor is essentially zero for these compounds. The relation of the acentric factor ω to Riedel's α reads ω = 4.93(α − 5.808), and therefore we limit ourselves further on to ω. The acentric factor ω is given for a variety of compounds in Appendix E [6].
(Figure 4.6). This apparently arbitrary definition was chosen because of the ease and precision with which this quantity can be determined (contrary to Riedel's α, which might be difficult to determine precisely). Since at T/Tcri = 0.7 the vapor pressure of simple fluids is close to 0.1 Pcri, the acentric factor is essentially zero for these compounds. The relation of the acentric factor ω to Riedel's α reads ω = 4.93(α − 5.808), and therefore we limit ourselves further on to ω. The acentric factor ω is given for a variety of compounds in Appendix E [6].
Figure 4.6 The acentric factor ω as the deviation of the slope of the vapor pressure of a normal from the fluid slope for simple fluids. Note: log = logarithm to base 10.
The simplest correlation [7] for normal fluids is
(4.14) ![]()
which correlates the reduced compression factor Z at critical conditions with the acentric factor ω. Pitzer et al. showed that, if we write
(4.15) ![]()
the second virial coefficient can be represented by
(4.16) ![]()
where B(0) and B(1) are functions of Tred, reasonably well described by
(4.17) ![]()
We thus have Z = Z(Tred,Pred,ω). More generally, the compression factor can be accurately described by Z = Z(0) + ωZ(1), where Z(0) and Z(1) are complex, extensively tabulated [8] functions of Tred. Alternatively, one uses a fit for ln Pred = f(0) + f(1)ω reading
(4.18) ![]()
(4.19) ![]()
For nonpolar fluids above 1 bar, the accuracy for Z is about 2–3%, but for more polar molecules and low pressures the calculated pressure is typically too low and the deviations are larger [6], perhaps up to 10%. It should be noted that these correlations typically apply only to normal fluids, as defined in Section 4.1.
Returning to the topic of the EoS, we note that other equations of state exist, for example, the Berthelot and Dieterici equations, given by, respectively,
(4.20) ![]()
As both equations contain only two parameters, they can both be scaled to a reduced equation of state, much like the vdW equation; however, similar to the vdW equation they are not very accurate.
More complex EoS are available, for example, the Redlich–Kwong (RK) [9], Redlich–Kwong–Soave (RKS) [10], Peng–Robinson (PR) [11], and Benedict–Webb–Rubin (BWR) [12] equations. These equations use as a general form
(4.21) ![]()
The original RK equation uses α(T) = T−1/2 and f(ρ) = 1 + bρ, still obeying the two-parameter PoCS. The RKS equation differentiates between various normal liquids by using the acentric factor in the expression for α(T), namely ![]() with m = 0.480 + 1.5574ω − ω2 in conjunction with f(ρ). The PR equation also modifies f(ρ) by using f(ρ) = 1 + 2bρ − b2ρ2, but with a slightly modified m = 0.37464 + 1.54226ω − 0.26992ω2. It appears that the RKS EoS is better suited to small values of ω, while the PR is better equipped for larger values of ω. Finally, we should mention the BWR equation and its modifications, in which the vdW repulsive term is replaced by a polynomial and exponential expression, the form being chosen also to be easily integrable to yield the Helmholtz energy. In its original form, the BWR equation uses eight parameters, but in order to maintain accuracy above 1.8ρcri it has been modified extensively. One particular attempt included 33 parameters [13].
with m = 0.480 + 1.5574ω − ω2 in conjunction with f(ρ). The PR equation also modifies f(ρ) by using f(ρ) = 1 + 2bρ − b2ρ2, but with a slightly modified m = 0.37464 + 1.54226ω − 0.26992ω2. It appears that the RKS EoS is better suited to small values of ω, while the PR is better equipped for larger values of ω. Finally, we should mention the BWR equation and its modifications, in which the vdW repulsive term is replaced by a polynomial and exponential expression, the form being chosen also to be easily integrable to yield the Helmholtz energy. In its original form, the BWR equation uses eight parameters, but in order to maintain accuracy above 1.8ρcri it has been modified extensively. One particular attempt included 33 parameters [13].
A compromise between accuracy and complexity is the general cubic EoS [14] of which the vdW equation is the prototype, although cubic equations of state have limited flexibility [15]. This equation reads for one mole (omitting the subscript, m)
(4.22) ![]()
with θ, η, κ, λ, and b as parameters. The vdW equation is obtained if η = b, θ = a, and κ = λ = 0. An often-used class is given by η = b, θ = a(T), κ = (ε + σ)b and λ = εσb2. In that case, the cubic equation reads
(4.23) ![]()
For this equation the parameters ε and σ have the same value for all substances in the class, while a(T) and b(T) are substance-dependent. The vdW equation is obtained by substituting a(T) = a and ε = σ = 0.
All of these equations can be developed in a power series in V in order to obtain the associated virial coefficients. For example, rewriting the van der Waals equation as
(4.24) ![]()
and expanding the first term on the right-hand side by the binomial series, we obtain
(4.25) ![]()
(4.26) ![]()
A similar procedure may be applied to other EoS. Experiments have shown that B2 < 0 at low temperature, becomes positive at higher temperature, reaches a maximum, and then decreases. The vdW equation gives, correctly, B2 < 0 for low temperature, changing to B2 > 0 at higher temperature, but does not predict a maximum.
The virial approach, however, can be applied without reference to any analytical empirical EoS by describing the experimentally determined pressure as a function of density directly with the virial series. It appears that this, initially fully empirical approach, can be rationalized by statistical thermodynamics, as shown in Chapter 5.
Problem 4.1
Discuss why the pressure correction in the vdW equation is proportional to (n/V)2. Also derive the hard sphere volume correction b = 4NA[4π(σ/2)3/3].
Problem 4.2
Show that for the vdW fluid the Boyle temperature TB, where the slightly nonideal fluid reacts as an ideal fluid and thus where B2(TB) = 0, is given by TB = a/Rb. Also show that the Joule–Thomson inversion temperature, characterizing the temperature TJT for which the Joule–Thomson coefficient μJT ≡ (∂T/∂P)H = V(αT − 1)/CP changes sign, hence the cooling changes to heating and vice versa, and where ∂(B2/T)/∂T = 0, is given by TJT = 2TB.
Problem 4.3
Show, by integrating the pressure P over the volume V, that the vdW expression P = RT/(V − b) − a/V2 yields the Helmholtz energy F(T,V) − F°(T,V) = − RTln[(V − b)/V°] − a/V, the entropy S(T,V) − S°(T,V) = Rln[(V − b)/V°], and the internal energy U(T,V) − U°(T,V) = −a/V, where F°, S° and U° refer to the perfect gas corresponding properties.
Problem 4.4
Show that the second and third virial coefficients of the EoS indicated are
|
Berthelot: |
B2 = b − a/RT2 |
B3 = b2 |
|
Dieterici: |
B2 = b − a/RT |
B3 = b2 − ab/RT + a2/2R2T2 |
Problem 4.5
Show that, if the virial coefficient is given by Bn, the coefficient in the corresponding Helmholtz expression reads ![]() .
.
Problem 4.6*
Show that the critical points for the EoSs and the values for Zcri indicated are:
![]()
Problem 4.7*
An equivalent procedure to obtain the vdW parameters is to use (V − Vcri)3 = 0, since at the critical point V = Vcri and the vdW equation is a cubic equation in V. Compare the expansion of this equation with the standard vdW equation in its polynomial form V3 − αV2 + βV − γ = 0. Show that 3Vcri = b + RTcri/Pcri, ![]() , and
, and ![]() . Show that from the last two equations it follows that
. Show that from the last two equations it follows that ![]() and b = Vcri/3. Also show that substitution of the expression for b in the first of these three equations allows solution for Vcri, which then can be eliminated from a and b to obtain Vcri = 3RTcri/8Pcri,
and b = Vcri/3. Also show that substitution of the expression for b in the first of these three equations allows solution for Vcri, which then can be eliminated from a and b to obtain Vcri = 3RTcri/8Pcri, ![]() , and b = RTcri/8Pcri.
, and b = RTcri/8Pcri.
Problem 4.8*
Show that ![]() , where ρ = N/V is the number density, and estimate its value for Ar, N2, and O2 at 0 °C and 1 atm. How large is this ratio for H2O at its equilibrium vapor pressure of 6.11 mbar at 0 °C?
, where ρ = N/V is the number density, and estimate its value for Ar, N2, and O2 at 0 °C and 1 atm. How large is this ratio for H2O at its equilibrium vapor pressure of 6.11 mbar at 0 °C?
References
1 (a) Tait, P.G. (1889) Voyages of HMS Challenger, vol. 2, part 4, HMSO, London, p. 1; (b) Dymond, J.H. and Malhotra, R. (1988) Int. J. Thermophys., 9, 941.
2 van der Waals, J.D. (1873) Over de Continuïteit van den Gas- en Vloeistoftoestand. Thesis.
3 Pitzer, K.S. (1929) J. Chem. Phys., 7, 583.
4 (a) Riedel, L. (1954) Chem.-Ing. Tech. Z., 26, 83, 259, 679; (b) Riedel, L. (1955) Chem.-Ing. Tech. Z., 27, 209, 475; (c) Riedel, L. (1956) Chem.-Ing. Tech. Z., 28, 557.
5 (a) Pitzer, K.S., Lippmann, D.Z., Curl, R.F., Jr, Huggins, C.M., and Petersen, D.E. (1955) J. Am. Chem. Soc., 77, 3433; (b) Pitzer, K.S. (1995) Appendix 3, in Thermodynamics, McGraw-Hill, New York.
6 For an extensive list, Reid, R.C., Prausnitz, J.M., and Poling, B.E. (1988) The Properties of Gases and Liquids, 4th edn, McGraw-Hill.
7 Schreiber, D.R. and Pitzer, K.S. (1989) Fluid Phase Eq., 46, 113.
8 Lee, B.I. and Kessler, M.G. (1975) AIChE J., 21, 510.
9 Redlich, O. and Kwong, J.N.S. (1949) Chem. Rev., 44, 233.
10 Soave, G. (1972) Chem. Eng. Sci., 27, 1197.
11 Peng, D.-Y. and Robinson, D.B. (1976) Ind. Eng. Chem. Fundam., 15, 59.
12 (a) Benedict, M., Webb, G.B., and Rubin, L.C. (1940) J. Chem. Phys., 8, 334; (b) Benedict, M., Webb, G.B., and Rubin, L.C. (1942) J. Chem. Phys., 10, 747.
13 Younglove, B.A. and Ely, J.F. (1987) J. Phys. Chem. Ref. Data, 16, 577.
14 Valderrama, J.O. (2003) Ind. Eng. Chem. Res., 42, 1603.
15 (a) Abbott, M.M. (1973) AIChE J., 19, 596; (b) Chao, K.C and Robinson, R.C., Jr (eds) (1979) Advances in Chemistry, vol. 182, American Chemical Society, Washington, DC, p. 47.
16 Finn, C.B.P. (1993) Thermal Physics, Chapman & Hall, London.
17 Sen, G.-J. (1946) Ind. Eng. Chem., 38, 803.
18 Moelwyn-Hughes, E.A. (1961) Physical Chemistry, 2nd edn, Pergamon, Oxford.
19 Guggenheim, E.A. (1967) Thermodynamics, 5th edn, North-Holland, Amsterdam.
Further Reading
Murrell, J.N. and Jenkins, A.D. (1994) Properties of Liquids and Solutions, 2nd edn, John Wiley & Sons, Ltd, Chichester.
Pryde, J.A. (1966) The Liquid State, Hutchinson University Library, London.
Rowlinson, J.S. and Swinton, F.L. (1982) Liquids and Liquid Mixtures, 3rd edn, Butterworth, London.