MCAT General Chemistry Review
Chapter 2: The Periodic Table
Conclusion
Now that we have completed our review of the Periodic Table of the Elements, commit to understanding (not just memorizing) the trends of physical and chemical properties of the elements. They will help you quickly answer many questions on the MCAT. As you progress through the chapters of this book, a foundational understanding of the elements will help you develop a richer, more nuanced understanding of their general and particular behaviors. Topics in general chemistry that may have given you trouble in the past will be understandable from the perspective of the behaviors and characteristics that you have reviewed here.
More broadly, you will see a diverse array of elements from the groups we have discussed here that are critical or detrimental to biological function. In addition, you may begin to see why the human body utilizes certain elements for specific purposes, taking advantage of the periodic trends discussed here.
Concept Summary
The Periodic Table
· The Periodic Table of the Elements organizes the elements according to their atomic numbers and reveals a pattern of similar chemical and physical properties among elements.
o Rows are called periods and are based on the same principal energy level, n.
o Columns are called groups. Elements in the same group have the same valence shell electron configuration.
Types of Elements
· The elements on the Periodic Table belong to one of three types.
o Metals are shiny (lustrous), conduct electricity well, and are malleable and ductile. Metals are found on left side and middle of the Periodic Table.
o Nonmetals are dull, poor conductors of electricity, and are brittle. Nonmetals are found on right side of the Periodic Table.
o Metalloids possess characteristics of both metals and nonmetals and are found in a stair-step pattern starting with boron (B).
Periodic Properties of the Elements
· Effective nuclear charge (Zeff) is the net positive charge experienced by electrons in the valence shell and forms the foundation for all periodic trends.
o Zeff increases from left to right across a period, with little change in value from top to bottom in a group.
o Valence electrons become increasingly separated from the nucleus as the principal energy level, n, increases from top to bottom in a group.
· Atomic radius decreases from left to right across a period and increases from top to bottom in a group.
· Ionic radius is the size of a charged species. The largest nonmetallic ionic radii and the smallest metallic ionic radii exist at the metalloid boundary.
o Cations are generally smaller than their corresponding neutral atom.
o Anions are generally larger than their corresponding neutral atom.
· Ionization energy is the amount of energy necessary to remove an electron from the valence shell of a gaseous species; it increases from left to right across a period and decreases from top to bottom in a group.
· Electron affinity is the amount of energy released when a gaseous species gains an electron in its valence shell; it increases from left to right across a period and decreases from top to bottom in a group.
· Electronegativity is a measure of the attractive force of the nucleus for electrons within a bond; it increases from left to right across a period and decreases from top to bottom in a group.
The Chemistry of Groups
· Alkali metals typically take on an oxidation state of +1 and prefer to lose an electron to achieve a noble gas-like configuration; they and the alkaline earth metals are the most reactive of all metals.
· Alkaline earth metals take on an oxidation state of +2 and can lose two electrons to achieve noble gas-like configurations.
· Chalcogens take on oxidation states of −2 or +6 (depending on whether they are nonmetals or metals, respectively) in order to achieve noble gas configuration. They are very biologically important.
· Halogens typically take on an oxidation state of −1 and prefer to gain an electron to achieve noble gas-like configurations; these nonmetals have the highest electronegativities.
· Noble gases have a fully filled valence shell in their standard state and prefer not to give up or take on additional electrons; they have very high ionization energies and (for He, Ne, and Ar), virtually nonexistent electronegativities and electron affinities.
· Transition metals are unique because they take on multiple oxidation states, which explains their ability to form colorful complexes with nonmetals in solution and their utility in certain biological systems.
Answers to Concept Checks
· 2.1
1. The modern Periodic Table is arranged in order by atomic number.
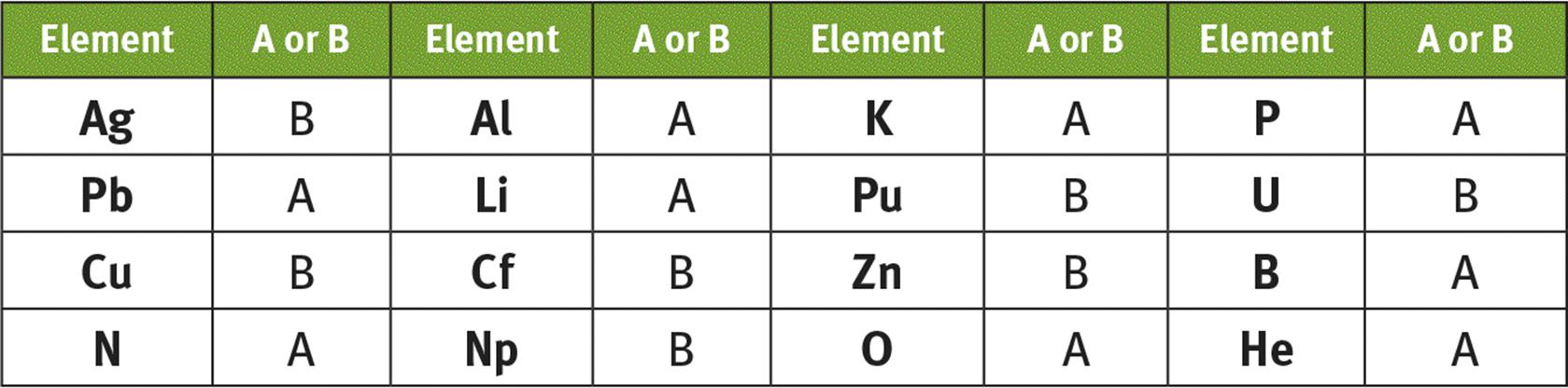
2. 
· 2.2
1. Metals have luster. Nonmetals have poor conductivity. Metalloids exhibit brittleness but good conductivity. Any answers within each of these categories are acceptable.
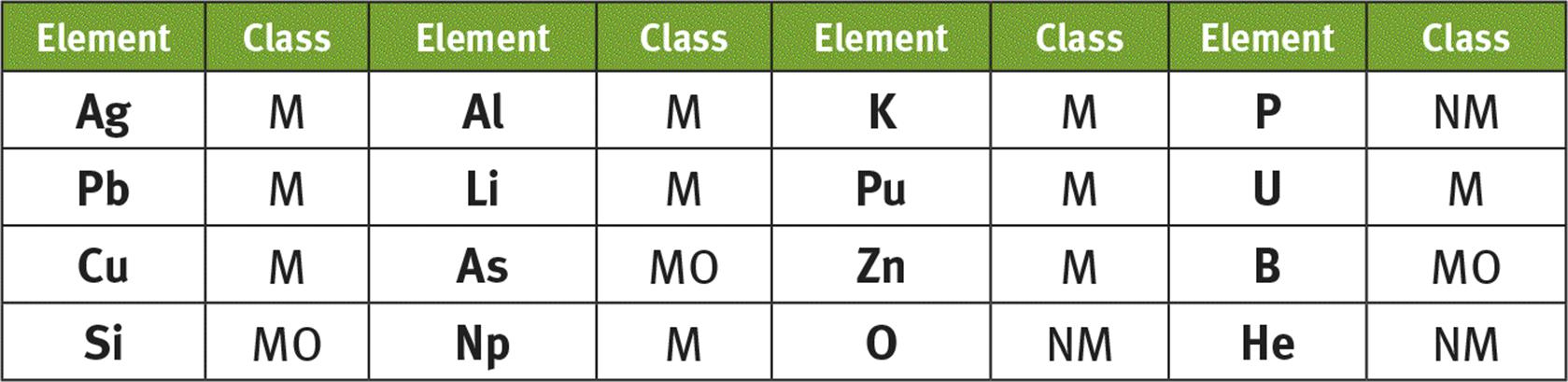
2. 
· 2.3
1. Ionization energy: carbon > germanium > calcium > potassium
2. Electron affinity: barium < yttrium < copper < sulfur
3. Electronegativity: oxygen > antimony > thallium > neon
4. Atomic radius: xenon < niobium < tantalum < praseodymium
· 2.4
1.
· High reactivity to water: Groups 1 and 2
· Six valence electrons: Groups 6 and 16
· Contain at least one metal: Groups 1 through 15
· Multiple oxidation states: All groups; most notably Groups 3 through 12 (transition metals)
· Negative oxidation states: Almost all groups; most notably Groups 14 through 17 (nonmetals)
· Possess a full octet in the neutral state: Group 18
Shared Concepts
· General Chemistry Chapter 1
o Atomic Structure
· General Chemistry Chapter 3
o Bonding and Chemical Interactions
· General Chemistry Chapter 4
o Compounds and Stoichiometry
· Organic Chemistry Chapter 3
o Bonding
· Physics and Math Chapter 5
o Electrostatics and Magnetism
· Physics and Math Chapter 9
o Atomic and Nuclear Phenomena