MCAT General Chemistry Review
Chapter 1: Atomic Structure
1.1 Subatomic Particles
Although you may have encountered in your university-level chemistry classes such subatomic particles as quarks, leptons, and gluons, the MCAT’s approach to atomic structure is much simpler. There are three subatomic particles that you must understand: protons, neutrons, and electrons.
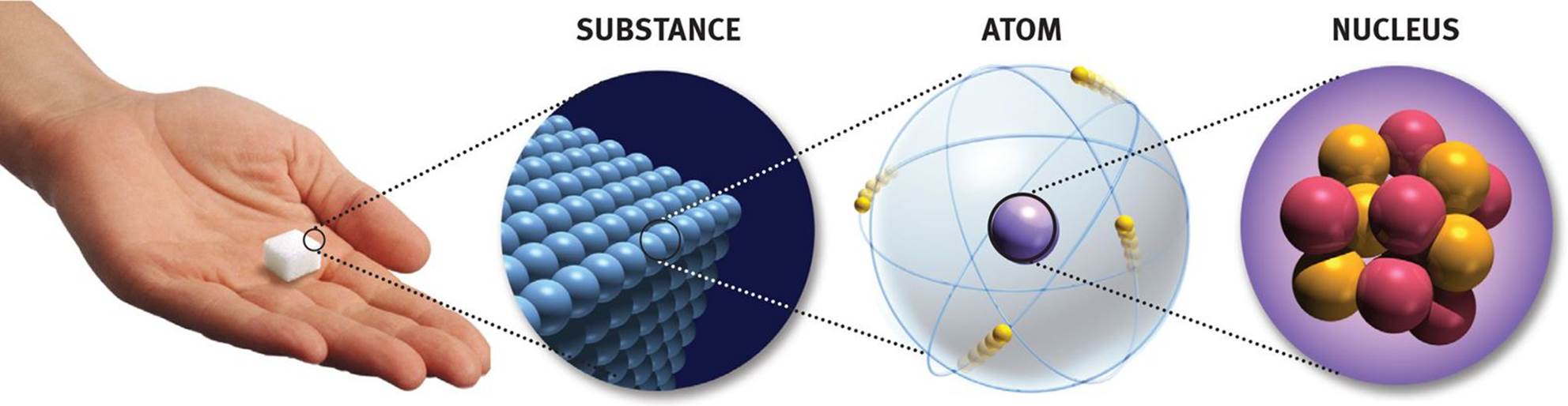 Figure 1.1. Matter: From Macroscopic to Microscopic
Figure 1.1. Matter: From Macroscopic to Microscopic
PROTONS
Protons are found in the nucleus of an atom, as shown in Figure 1.1. Each proton has an amount of charge equal to the fundamental unit of charge (e = 1.6 × 10−19 C), and we denote this fundamental unit of charge as “+1 e” or simply “+1” for the proton. Protons have a mass of approximately one atomic mass unit (amu). The atomic number (Z) of an element, as shown in Figure 1.2, is equal to the number of protons found in an atom of that element. As such, it acts as a unique identifier for each element because elements are defined by the number of protons they contain. For example, all atoms of oxygen contain eight protons; all atoms of gadolinium contain 64 protons. While all atoms of a given element have the same atomic number, they do not necessarily have the same mass—as we will see in our discussion of isotopes.
 Figure 1.2. Potassium, from the Periodic Table Potassium has the symbol K (Latin: kalium), atomic number 19, and atomic weight of approximately 39.1.
Figure 1.2. Potassium, from the Periodic Table Potassium has the symbol K (Latin: kalium), atomic number 19, and atomic weight of approximately 39.1.
NEUTRONS
Neutrons, as the name implies, are neutral—they have no charge. A neutron’s mass is only slightly larger than that of the proton, and together, the protons and the neutrons of the nucleus make up almost the entire mass of an atom. Every atom has a characteristic mass number (A), which is the sum of the protons and neutrons in the atom’s nucleus. A given element can have a variable number of neutrons; thus, while atoms of the same element always have the same atomic number, they do not necessarily have the same mass number. Atoms that share an atomic number but have different mass numbers are known as isotopes of the element, as shown in Figure 1.3. For example, carbon (Z = 6) has three naturally occurring isotopes: 612C, with six protons and six neutrons; 613C, with six protons and seven neutrons; and 614C, with six protons and eight neutrons. The convention ZAX is used to show both the atomic number (Z) and the mass number (A) of atom X.
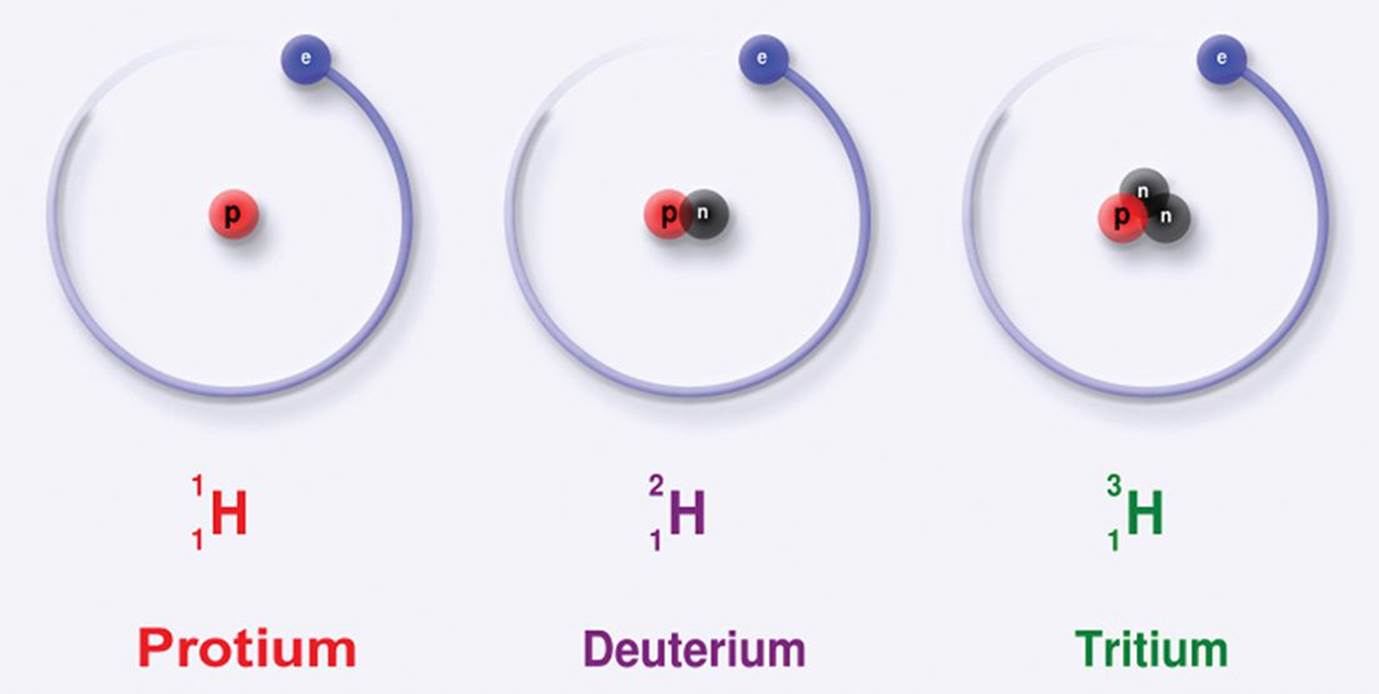 Figure 1.3. Various Isotopes of Hydrogen Atoms of the same element have the same atomic number (Z = 1), but may have varying mass numbers (Az = 1, 2, or 3).
Figure 1.3. Various Isotopes of Hydrogen Atoms of the same element have the same atomic number (Z = 1), but may have varying mass numbers (Az = 1, 2, or 3).
ELECTRONS
Electrons move through the space surrounding the nucleus and are associated with varying levels of energy. Each electron has a charge equal in magnitude to that of a proton, but with the opposite (negative) sign, denoted by “−1 e” or simply “–e.” The mass of an electron is approximately ![]() that of a proton. Because subatomic particles’ masses are so small, the electrostatic force of attraction between the unlike charges of the proton and electron is far greater than the gravitational force of attraction based on their respective masses.
that of a proton. Because subatomic particles’ masses are so small, the electrostatic force of attraction between the unlike charges of the proton and electron is far greater than the gravitational force of attraction based on their respective masses.
Electrons move around the nucleus at varying distances, which correspond to varying levels of electrical potential energy. The electrons closer to the nucleus are at lower energy levels, while those that are further out (in higher shells) have higher energy. The electrons that are farthest from the nucleus have the strongest interactions with the surrounding environment and the weakest interactions with the nucleus. These electrons are called valence electrons; they are much more likely to become involved in bonds with other atoms because they experience the least electrostatic pull from their own nucleus. Generally speaking, the valence electrons determine the reactivity of an atom. As we will discuss in Chapter 3 of MCAT General Chemistry Review, the sharing of these valence electrons in covalent bonds allows elements to fill their highest energy level to increase stability. In the neutral state, there are equal numbers of protons and electrons; losing electrons results in the atom gaining a positive charge, while gaining electrons results in the atom gaining a negative charge. A positively charged atom is called a cation, and a negatively charged atom is called an anion.
BRIDGE
Valence electrons will be very important to us in both general and organic chemistry. Knowing how tightly held those electrons are will allow us to understand many of an atom’s properties and how it interacts with other atoms, especially in bonding. Bonding is so important that it is discussed in Chapter 3 of both MCAT General Chemistry Review and MCAT Organic Chemistry Review.
Some basic features of the three subatomic particles are shown in Table 1.1.
|
Subatomic Particle |
Symbol |
Relative Mass |
Charge |
Location |
|
Proton |
p, p+, or 11H |
1 |
+1 |
Nucleus |
|
Neutron |
n0 or 01n |
1 |
0 |
Nucleus |
|
Electron |
e− or −10e |
0 |
−1 |
Orbitals |
|
Table 1.1. Subatomic Particles |
||||
Example:
Determine the number of protons, neutrons, and electrons in a nickel-58 atom and in a nickel-60 +2 cation.
Solution:
58Ni has an atomic number of 28 and a mass number of 58. Therefore, 58Ni will have 28 protons, 28 electrons, and 58 – 28, or 30, neutrons.
60Ni2+ has the same number of protons as the neutral 58Ni atom. However, 60Ni2+ has a positive charge because it has lost two electrons; thus, Ni2+ will have 26 electrons. Also, the mass number is two units higher than for the 58Ni atom, and this difference in mass must be due to two extra neutrons; thus, it has a total of 32 neutrons.
MCAT Concept Check 1.1:
Before you move on, assess your understanding of the material with these questions.
1. Which subatomic particle is the most important for determining each of the following properties of an atom?
· Charge:
· Atomic number:
· Isotope:
2. In nuclear medicine, isotopes are created and used for various purposes; for instance, 18O is created from 18F. Determine the number of protons, neutrons, and electrons in each of these species.
|
Particle |
Protons |
Neutrons |
Electrons |
|
18O |
|||
|
18F |