MCAT General Chemistry Review
Chapter 6: Equilibrium
Practice Questions
1. A reaction is found to stop just before all reactants are converted to products. Which of the following could be true about this reaction?
1. The reaction is irreversible, and the forward rate is greater than the reverse rate.
2. The reaction is irreversible, and the reverse rate is too large for products to form.
3. The reaction is reversible, and the forward rate is equal to the reverse rate.
4. The reaction is reversible, and the reverse rate is greater than the forward rate.
2. What is the equilibrium expression for the reaction Cu2SO4 (s) ⇌ 2 Cu+ (aq) + SO42− (aq)?
1. 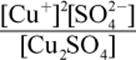
2. 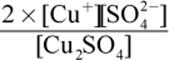
3. ![]()
4. ![]()
3. Carbonated beverages are produced by dissolving carbon dioxide in water to produce carbonic acid:
CO2 (g) + H2O (l) ⇌ H2CO3 (aq)
When a bottle containing carbonated water is opened, the taste of the beverage gradually changes as the carbonation is lost. Which of the following statements best explains this phenomenon?
1. The change in pressure and volume causes the reaction to shift to the left, thereby decreasing the amount of aqueous carbonic acid.
2. The change in pressure and volume causes the reaction to shift to the right, thereby decreasing the amount of gaseous carbon dioxide.
3. Carbonic acid reacts with environmental oxygen and nitrogen.
4. Carbon dioxide reacts with environmental oxygen and nitrogen.
4. What is the proper equilibrium expression for the reaction below?
2 NO2 (g) + 4 H2 (g) ⇌ N2 (g) + 4 H2O (g)
1. 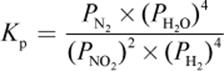
2. 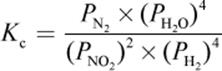
3. 
4. 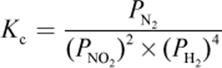
5. If Kc ≫ 1:
1. the equilibrium mixture will favor products over reactants.
2. the equilibrium mixture will favor reactants over products.
3. the equilibrium concentrations of reactants and products are equal.
4. the reaction is essentially irreversible.
6. Acetic acid dissociates in solution according to the following equation:
CH3COOH ⇌ CH3COO– + H+
If sodium acetate is added to a solution of acetic acid in excess water, which of the following effects would be observed in the solution?
1. Decreased pH
2. Increased pH
3. Decreased pKeq (pKa)
4. Increased pKeq (pKa)
7. Given the reaction below:
FeI (aq) + I2 (g) → FeI3 (aq)
Which of the following would increase the formation of product?
1. Decreasing the volume of the container
2. Decreasing the pressure of the container
3. Increasing the volume of the container
4. Decreasing the volume of the container while maintaining a constant pressure
8. If the reaction in question 7 were exothermic, what effect would decreasing the temperature have on the equilibrium?
1. The forward reaction rate and the reverse reaction rate both increase.
2. The forward reaction rate decreases while the reverse reaction rate increases.
3. The forward reaction rate increases while the reverse reaction rate decreases.
4. The forward reaction rate and the reverse reaction rate both decrease.
9. Which of the following actions does NOT affect the equilibrium position of a reaction?
1. Adding or subtracting heat.
2. Adding or removing a catalyst.
3. Increasing or decreasing concentrations of reactants.
4. Increasing or decreasing volumes of reactants.
10.In a sealed 1 L container, 1 mole of nitrogen gas reacts with 3 moles of hydrogen gas to form 0.05 moles of NH3 at equilibrium. Which of the following is closest to the Kc of the reaction?
1. 0.0001
2. 0.001
3. 0.01
4. 0.1
11.Increasing temperature can alter the Keq of a reaction. Why might increasing temperature indefinitely be unfavorable for changing reaction conditions?
1. The equilibrium constant has a definite limit that cannot be surpassed.
2. The products or reactants can decompose at high temperatures.
3. Increasing temperature would decrease pressure, which may or may not alter reaction conditions.
4. If a reaction is irreversible, its Keq will resist changes in temperature.
12.Which of the following is true of equilibrium reactions?
1. An increase in k1 results in a decrease in k–1.
2. As the concentration of products increases, the concentrations of reactants decreases.
3. The equilibrium constant is altered by changes in temperature.
1. I only
2. II and III only
3. I and III only
4. I, II, and III
13.Compound A has a Ka (equilibrium constant of acid dissociation) of approximately 10–4. Which of the following compounds is most likely to react with a solution of compound A?
1. HNO3
2. NO2
3. NH3
4. N2O5
14.Consider the following two reactions:
3 A + 2 B ⇌ 3 C + 4 D (Reaction 1)
4 D + 3 C ⇌ 3 A + 2 B (Reaction 2)
If Keq for reaction 1 is equal to 0.1, what is Keq for reaction 2?
1. 0.1
2. 1
3. 10
4. 100
15.Which of the following statements best describes the effect of lowering the temperature of the following reaction?
![]()
1. [C] and [D] would increase.
2. [A] and [B] would increase.
3. ΔH would increase.
4. ΔH would decrease.
PRACTICE QUESTIONS