MCAT General Chemistry Review
Chapter 8: The Gas Phase
8.1 The Gas Phase
Matter can exist in three different physical forms, called phases or states: gas, liquid, and solid. We have discussed liquids in the context of intermolecular forces and solids in the context of organized crystals in Chapter 3 of MCAT General Chemistry Review. The gaseous phase may be the simplest to understand because all gases display similar behavior and follow similar laws regardless of their particular chemical identities. Like liquids, gases are classified as fluids because they can flow and take on the shapes of their containers. However, the atoms or molecules in a gaseous sample move rapidly and are far apart from each other. In addition, only very weak intermolecular forces exist between gas particles; this results in certain characteristic physical properties, such as the ability to expand to fill any volume. Gases are also easily—although not infinitely—compressible, which distinguishes them from liquids.
VARIABLES
We can define the state of a gaseous sample by four variables: pressure (P), volume (V), temperature (T), and number of moles (n).
Gas pressures are usually expressed in units of atmospheres (atm) or in millimeters of mercury (mmHg), which are equivalent to torr. The SI unit for pressure, however, is the pascal (Pa). The mathematical relationships among all of these units are as follows:
1 atm = 760 mmHg ≡ 760 torr = 101.325 kPa
Medical devices that measure blood pressure are termed sphygmomanometers, and the most clinically relevant unit of measurement for them is mmHg. In fact many medical devices utilize the same conceptual design of a barometer, shown in Figure 8.1, to continuously monitor blood pressure.
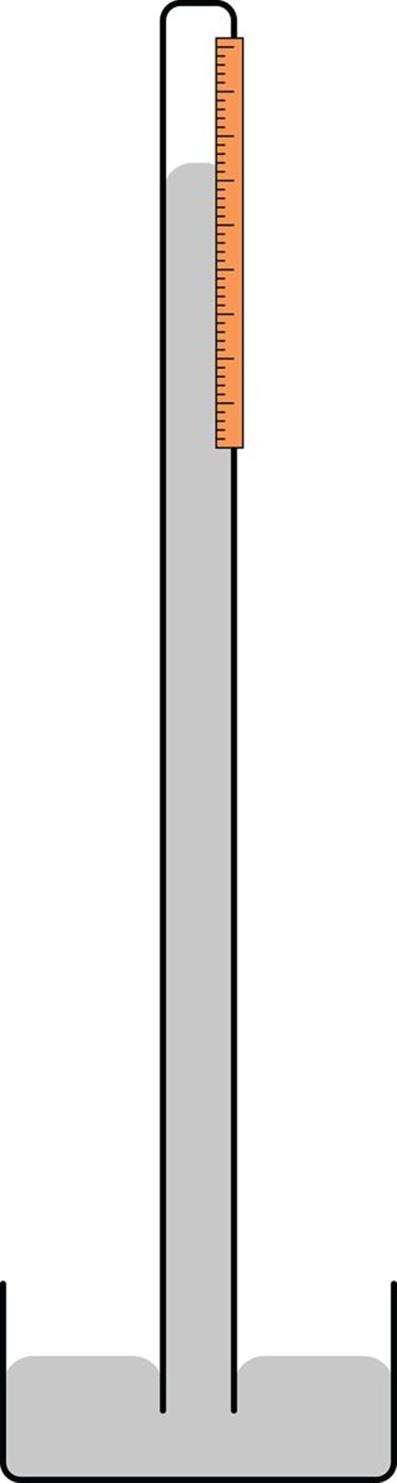 Figure 8.1. Schematic of a Simple Mercury Barometer
Figure 8.1. Schematic of a Simple Mercury Barometer
In order to explain why the mercury rises in a barometer, we must summarize the forces at play here. Atmospheric pressure creates a downward force on the pool of mercury at the base of the barometer while the mercury in the column exerts an opposing force (its weight) based on its density. The weight of the mercury creates a vacuum in the top of the tube. When the external air exerts a higher force than the weight of the mercury in the column, the column rises. When the external air exerts a lower force than the weight of the mercury, the column falls. Thus, a reading can be obtained by measuring the height of the mercury column (in mm), which will be directly proportional to the atmospheric pressure being applied.
BRIDGE
Fluid dynamics is an important concept discussed in Chapter 4 of MCAT Physics and Math Review that applies to multiple aspects of the gas laws covered here, including the functionality of a mercury barometer.
It is important to mention here that atmospheric pressure is not the only external pressure that can exert this force. For instance, a clinical blood pressure cuff creates a force that is opposed by the person’s systolic and diastolic arterial blood pressure.
REAL WORLD
Blood pressure is measured by a sphygmomanometer, which uses units of mmHg. A normal adult blood pressure is considered less than 120 mmHg systolic and 80 mmHg diastolic (< 120/80). Hypertension (high blood pressure) is defined as having at least two blood pressure readings > 140 mmHg systolic or > 90 mmHg diastolic.
The volume of a gas is generally expressed in liters (L) or milliliters (mL). Temperature is usually given in kelvin (K), although Celsius (°C) may be used instead. Many processes involving gases take place under standard temperature and pressure (STP), which refers to conditions of 273 K (0°C) and 1 atm.
A note of caution: STP conditions are not identical to standard state conditions. The two standards involve different temperatures and are used for different purposes. STP (273 K and 1 atm) is generally used for gas law calculations; standard state conditions (298 K, 1 atm, 1 Mconcentrations) are used when measuring standard enthalpy, entropy, free energy changes, and electrochemical cell voltage.
MCAT EXPERTISE
On the MCAT, remember that STP is different from standard state. Temperature at STP is 0°C (273 K). Temperature at standard state is 25°C (298 K).
MCAT Concept Check 8.1:
Before you move on, assess your understanding of the material with these questions.
1. Name some characteristics that make the gas phase unique:
2. A mercury barometer is primarily affected by atmospheric pressure. What would happen to the level of the mercury in the column if:
· the barometer was moved to the top of a mountain?
· the barometer was placed ten meters under water?
3. What are the conditions for STP?
4. What are the standard conditions?