MCAT General Chemistry Review
Chapter 8: The Gas Phase
Practice Questions
1. Which of the following sets of conditions would be LEAST likely to result in ideal gas behavior?
1. High pressure and low temperature
2. Low temperature and large volume
3. High pressure and large volume
4. Low pressure and high temperature
2. What is the density of neon gas in ![]() at STP?
at STP?
1. 452.3
2. 226.0
3. 1.802
4. 0.9018
3. A leak of helium gas through a small hole occurs at a rate of ![]() How will the leakage rates of neon and oxygen gases compare to helium at the same temperature and pressure?
How will the leakage rates of neon and oxygen gases compare to helium at the same temperature and pressure?
1. Neon will leak faster than helium; oxygen will leak faster than helium.
2. Neon will leak faster than helium; oxygen will leak slower than helium.
3. Neon will leak slower than helium; oxygen will leak faster than helium.
4. Neon will leak slower than helium; oxygen will leak slower than helium.
4. A 0.040 g piece of magnesium is placed in a beaker of hydrochloric acid. Hydrogen gas is generated according to the following equation:
Mg (s) + 2 HCl (aq) → MgCl2 (aq) + H2 (g)
The gas is collected over water at 25°C, and the gauge pressure during the experiment reads 784 mmHg.
The gas displaces a volume of 100 mL. The vapor pressure of water at 25°C is approximately 24.0 mmHg. Based on this data, how many moles of hydrogen are produced in this reaction? (Note:
![]()
1. 4.04 × 10−5 moles hydrogen
2. 4.09 × 10−3 moles hydrogen
3. 3.07 × 10−2 moles hydrogen
4. 3.11 moles hydrogen
5. Ideal gases:
1. have no volume.
2. have particles with no attractive forces between them.
3. have no mass.
1. I only
2. II only
3. I and II only
4. I, II, and III
6. An 8.00 g sample of NH4NO3 (s) is placed into an evacuated 10 L flask and heated to 227°C. After the NH4NO3 completely decomposes, what is the approximate pressure in the flask?
NH4NO3 (s) → N2O (g) + H2O (g)
1. 0.410 atm
2. 0.600 atm
3. 0.821 atm
4. 1.23 atm
7. The kinetic molecular theory states that:
1. the average kinetic energy of a molecule of gas is directly proportional to the temperature of the gas in kelvin.
2. collisions between gas molecules are inelastic.
3. gas particles occupy discrete areas of space.
4. all gas molecules have the same kinetic energy at the same temperature.
8. The plots of two gases at STP are shown below. One of the gases is 1.0 L of helium, and the other is 1.0 L of bromine. Which plot corresponds to each gas and why?
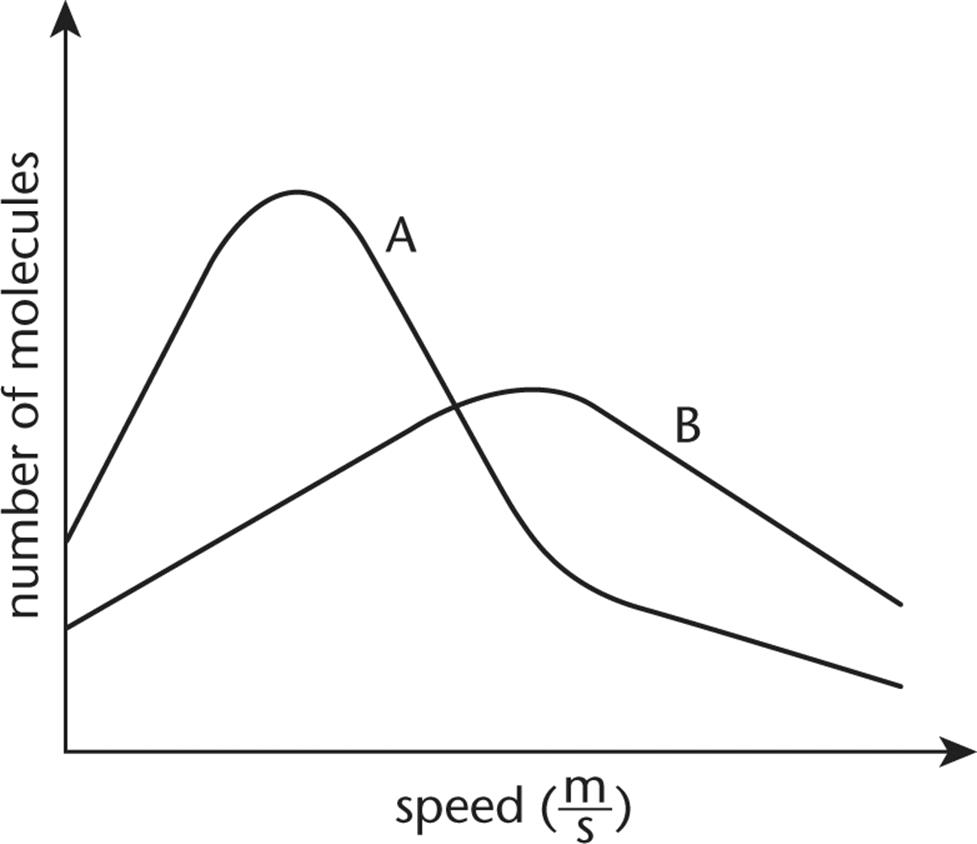
1. Curve A is helium and curve B is bromine because helium has a smaller molar mass than bromine.
2. Curve A is helium and curve B is bromine because the average kinetic energy of bromine is greater than the average kinetic energy of helium.
3. Curve A is bromine and curve B is helium because helium has a smaller molar mass than bromine.
4. Curve A is bromine and curve B is helium because the average kinetic energy of bromine is greater than the average kinetic energy of helium.
9. A balloon at standard temperature and pressure contains 0.20 moles of oxygen and 0.60 moles of nitrogen. What is the partial pressure of oxygen in the balloon?
1. 0.20 atm
2. 0.25 atm
3. 0.33 atm
4. 0.80 atm
10.Given that the gases at the center of the sun have an average molar mass of ![]() compressed to a density of
compressed to a density of ![]() under 1.30 × 109 atm of pressure, what is the temperature at the center of the sun?
under 1.30 × 109 atm of pressure, what is the temperature at the center of the sun?
1. 2.6 × 104 K
2. 2.6 × 106 K
3. 2.6 × 107 K
4. 2.6 × 1010 K
11.The gaseous state of matter is characterized by which of the following properties?
1. Gases are compressible.
2. Gases assume the volume of their containers.
3. Gas particles exist as diatomic molecules.
1. I only
2. I and II only
3. II and III only
4. I, II, and III
12.A gas at a temperature of 27°C has a volume of 60.0 mL. What temperature change is needed to increase this gas to a volume of 90.0 mL?
1. A reduction of 150°C
2. An increase of 150°C
3. A reduction of 40.5°C
4. An increase of 40.5°C
13.A gaseous mixture contains nitrogen and helium and has a total pressure of 150 torr. The nitrogen particles comprise 80 percent of the gas, and the helium particles make up the other 20 percent of the gas. What is the pressure exerted by each individual gas?
1. 100 torr nitrogen, 50.0 torr helium
2. 120 torr nitrogen, 30.0 torr helium
3. 30.0 torr nitrogen, 150 torr helium
4. 50.0 torr nitrogen, 100 torr helium
14.In which of the following situations is it impossible to predict how the pressure will change for a gas sample?
1. The gas is cooled at a constant volume.
2. The gas is heated at a constant volume.
3. The gas is heated, and the volume is simultaneously increased.
4. The gas is cooled, and the volume is simultaneously increased.
15.Experimenters notice that the molar concentration of dissolved oxygen in an enclosed water tank has decreased to one-half its original value. In an attempt to counter this decrease, they quadruple the partial pressure of oxygen in the container. What is the final concentration of the gas?
1. Half the original concentration
2. The same as the original concentration
3. Double the original concentration
4. Quadruple the original concentration
PRACTICE QUESTIONS