MCAT General Chemistry Review
Chapter 10: Acids and Bases
Answers and Explanations
1. DA Brønsted–Lowry base is defined as a proton acceptor. Ammonia, fluoride, and water—choices (A), (B), and (C), respectively—each accept a proton. Choice (D), HNO2, is a far better Brønsted–Lowry acid, donating a proton to solution.
2. BFirst, convert the concentration to 5 × 10−3 M. Next, because sulfuric acid is a strong acid, we can assume that, for the majority of sulfuric acid molecules (although not all), both protons will dissociate. The concentration of hydrogen ions is therefore 2 × 5 × 10−3, or 10−2. The equation for pH is pH = −log [H+]. If [H+] = 10−2 M, then pH = 2.
3. AAnswering this question is simply a matter of knowing nomenclature. Acids ending in –ic are derivatives of anions ending in –ate, while acids ending in –ous are derivatives of anions ending in –ite. ClO3−, choice (B), is chlorate because it has more oxygen than the other commonly occurring ion, ClO2−, which is named chlorite. Therefore, HClO3 is chloric acid. HClO2, choice (C), represents chlorous acid. HClO, choice (D), represents hypochlorous acid.
4. BSoluble hydroxides of Group IA and IIA metals are strong bases, eliminating choices (A) and (D). Choices (B) and (C) are both weak bases; however, methylamine contains an alkyl group, which is electron-donating. This increases the electron density on the nitrogen in methylamine, making it a stronger (Lewis) base. Therefore, ammonia is the weakest base.
5. BThe purpose of a buffer is to resist changes in the pH of a reaction. Buffers are not generally used to affect the kinetics of a reaction, so choices (C) and (D) are incorrect. Choice (A) is correct only in specific circumstances where the pH of the buffer solution itself is neutral. Many natural buffer systems maintain pH in the acidic or basic ranges.
6. D
The question is asking for pH, but because of the information given, we must first find the pOH and then subtract it from 14 to get the pH. Use the Henderson–Hasselbalch equation:

If the pOH = 2.45, the pH = 14 − 2.45 = 11.55.
7. AThe first pKa in this curve can be estimated by eye. It is located halfway between the starting point (when no base had yet been added) and the first equivalence point (the first steep portion of the graph, around 15 mL). This point is at approximately 7–8 mL on the x-axis, which corresponds to a pH of approximately 1.9. Notice that this region experiences very little change in pH, which is the defining characteristic of a buffer region.
8. CThe second equivalence point is the midpoint of the second steep increase in slope. This corresponds to approximately pH = 5.9.
9. BThe value of the second pKa is found at the midpoint between the first and second equivalence points. In this curve, that corresponds to pH = 4.1. Just like the first pKa, it is in the center of a flat buffering region.
10.BGram equivalent weight is the weight (in grams) that releases 1 acid or base equivalent from a compound. Because H3PO4 contains 3 protons, we find the gram equivalent weight by dividing the mass of one mole of the species by 3. The molar mass of phosphoric acid is ![]() so the gram equivalent weight is 32.7 g.
so the gram equivalent weight is 32.7 g.
11.D
This question requires the application of the acid dissociation formula. Weak acids do not dissociate completely; therefore, all three species that appear in the balanced equation will be present in solution. Hydrogen ions and conjugate base anions dissociate in equal amounts, so [H+] = [XO2−]. If the initial concentration of HXO2 was 2 M and some amount x dissociates, we will have x amount of H3O+ and XO2− at equilibrium, with 2 M − x amount of HXO2 at equilibrium.
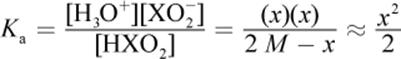
Note that x was considered negligible when added or subtracted, per usual. Solving for x, we get:
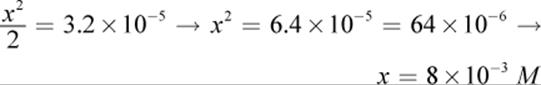
12.CA higher Ka implies a stronger acid. Weak acids usually have a Ka that is several orders of magnitude below 1. The pKa of a compound is the pH at which there are equal concentrations of acid and conjugate base; the pKa of this compound would be −log 1 = 0. With such a low pKa, this compound must be an acid. Therefore, the pH of any concentration of this compound must be below 7.
13.DAn amphoteric species is one that can act either as an acid or a base, depending on its environment. Proton transfers are classic oxidation–reduction reactions, so choices (A) and (B) are true. Choice (C) is true because many amphoteric species, such as water and bicarbonate, can either donate or accept a proton. Choice (D) is false, and thus the correct answer because amphoteric species can be either polar or nonpolar in nature.
14.CNaOH is a strong base; as such, there will be 1.2 × 10−5 M OH− in solution. Based on this information alone, the pOH must be between 4 and 5, and the pH must be between 9 and 10. Using the shortcut, pOH ≈ 5 − 0.12 = 4.88. pH = 14 − pOH = 9.12 (actual = 9.08).
15.CUse the equivalence point equation: NaVa = NbVb. Ba(OH)2 can dissociate to give two hydroxide ions, so its normality is 2 M × 2 = 4 N. H3PO4 can dissociate to give three hydronium ions, so its normality is 6 M × 3 = 18 N. Plugging into the equation, we get (18 N)(4 L) = (4 N)(Vb). Therefore, Vb is 18 L.