MCAT General Chemistry Review
Chapter 10: Acids and Bases
Practice Questions
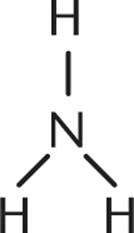
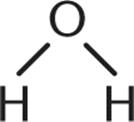
1. Which of the following is not a Brønsted–Lowry base?
1. 
2. F−
3. 
4. H — O — N = O
2. Which of the following is closest to the pH of a solution containing 5 mM H2SO4?
1. 1
2. 2
3. 3
4. 4
3. Which of the following represents chloric acid?
1. HClO3
2. ClO3−
3. HClO2
4. HClO
4. Which of the following bases is the weakest?
1. KOH
2. NH3
3. CH3NH2
4. Ca(OH)2
5. The function of a buffer is to:
1. maintain a neutral pH.
2. resist changes in pH when small amounts of acid or base are added.
3. slow down reactions between acids and bases.
4. speed up reactions between acids and bases.
6. What is the pH of a solution with an ammonium concentration of 70 mM and an ammonia concentration of 712 mM? (Note: The pKb of ammonia is 3.45.)
1. 2.45
2. 4.45
3. 9.55
4. 11.55
7.
1. Questions 7–9 refer to the titration curve of acid X shown below:
2. 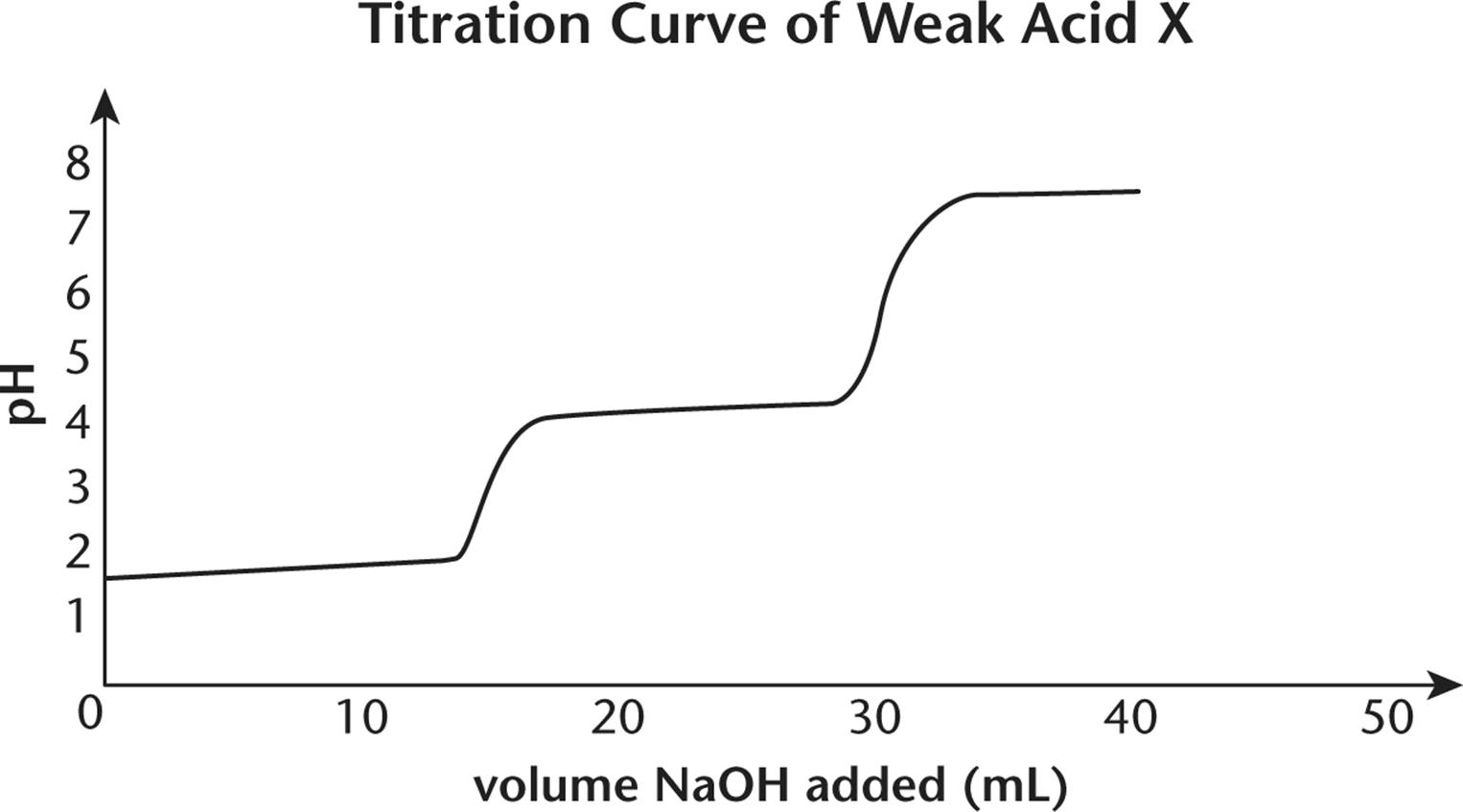
3. What is the approximate value of pKa1?
1. 1.9
2. 2.9
3. 3.8
4. 4.1
4. At what pH is the second equivalence point?
1. pH = 3.0
2. pH = 4.1
3. pH = 5.9
4. pH = 7.2
5. What is the approximate value of pKa2?
1. 3.6
2. 4.1
3. 5.5
4. 7.2
6. What is the gram equivalent weight of phosphoric acid?
1. 24.5 g
2. 32.7 g
3. 49.0 g
4. 98.0 g
7. What is the [H3O+] of a 2 M aqueous solution of a weak acid HXO2 with Ka = 3.2 × 10−5?
1. 6.4 × 10−5 M
2. 1.3 × 10−4 M
3. 4.0 × 10−3 M
4. 8.0 × 10−3 M
8. A solution is prepared with an unknown concentration of a theoretical compound with a Ka of exactly 1.0. What is the pH of this solution?
1. Higher than 7
2. Exactly 7
3. Less than 7
4. There is not enough information to answer the question.
9. Which of the following is NOT a characteristic of an amphoteric species?
1. Amphoteric species can act as an acid or a base, depending on its environment.
2. Amphoteric species can act as an oxidizing or reducing agent, depending on its environment.
3. Amphoteric species are sometimes amphiprotic.
4. Amphoteric species are always nonpolar.
10.What is the approximate pH of a 1.2 × 10−5 M aqueous solution of NaOH?
1. 4.92
2. 7.50
3. 9.08
4. 12.45
11.How many liters of 2 M Ba(OH)2 are needed to titrate a 4 L solution of 6 M H3PO4?
1. 1.33 L
2. 12 L
3. 18 L
4. 56 L
PRACTICE QUESTIONS