The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Thermodynamics
A. INTRODUCTION
In this chapter, the review will mainly focus on the laws of thermodynamics and their implications. The discussion includes concepts such as enthalpy, entropy, specific heat, heat capacity, Gibbs free energy, spontaneity of reactions, and related aspects. We have many ideas to discuss. So buckle up!
What is Thermodynamics?
In our busy modern life, we often forget about the fundamental aspects of today’s life that make it so unique. Everyday we use machines and gadgets, big and small, which depend on some type of energy. We harvest the available energy and use it in many ways. But how is this done? We use natural energy sources such as coal, petroleum products, and nuclear energy. The question is, what are the fundamental principles regarding the harvesting of energy from these sources? It is by chemical reactions that we make use of the hidden energy available from these sources. What we learn from thermodynamics is the dynamics of energy (heat) transfer during chemical changes. In this chapter, we will review the various aspects of thermodynamics and the related principles.
B. THE FIRST LAW OF THERMODYNAMICS
According to the first law of thermodynamics, energy is neither created nor destroyed, which also implies that the total energy present in the whole universe is constant. Based on this law, we can say that the energy is just taken from one form and converted to another form. This is exactly what we are doing when we are burning fuels such as gasoline to get sufficient energy to run the internal combustion engine, or when we are using nuclear reactions to turn the turbines of a nuclear power plant to make electricity.
Basic Aspects of Thermodynamics
First, let’s talk about internal energy. Internal energy of a substance is the total energy present in the particular quantity of that substance. For a chemical reaction or a physical change of reactants, the change in internal energy (Δ E) of the reactants is expressed as follows:
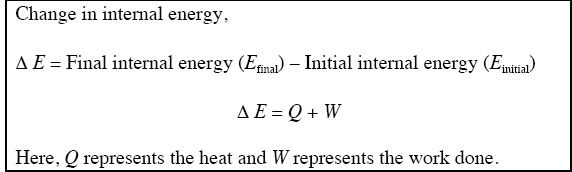
Based on the above equation, these conventions are followed:
When heat is absorbed by a system, heat (Q) is positive.
When heat is given off by a system, heat (Q) is negative.
When work is done on a system, work (W) is positive.
When work is done by a system, work (W) is negative.
C. ENDOTHERMIC AND EXOTHERMIC REACTIONS
The change in heat for chemical reactions at constant pressure is called enthalpy change, denoted by ΔH. The change in enthalpy (ΔH) of a reaction is represented as shown below:
![]()
If the reaction is endothermic, the change in enthalpy is positive. In an endothermic reaction, the enthalpy of the reactants is less than the enthalpy of the products. So the reaction is accomplished by supplying energy to the reaction. On the other hand, the change in enthalpy is negative if the reaction is exothermic. Energy is released in this case. Also it is worth remembering that in an exothermic reaction, the enthalpy of the reactants is greater than the enthalpy of the products. Hence, if we have the enthalpy data, we can predict whether the reaction is endothermic or exothermic.
D. LAW OF HEAT SUMMATION
According to the law of heat summation put forth by Henri Hess, we can find the total enthalpy of a reaction by adding the enthalpy changes of the individual steps in a reaction. It doesn’t really matter if the reaction occurs in many steps or in one step. As long as the enthalpy values that we sum up are correct, we will get the overall enthalpy change.
Law of heat summation: The total enthalpy of a reaction can be derived by adding the enthalpy changes of the individual steps in the reaction.
Example 10-1
Calculate the ΔH of the reaction between HCl and fluorine, forming HF and chlorine. The enthalpies of the related reactions are also given.
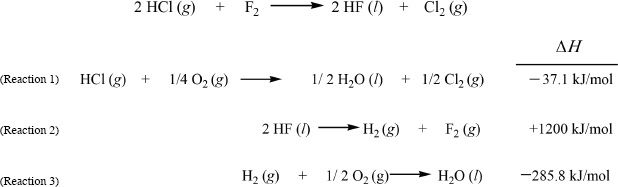
Solution:
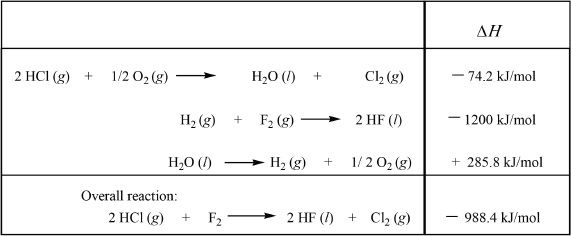
The individual reactions that are given have to be reversed if necessary, so that the sum of the sub-reactions will add up to the overall equation. Reaction 1 should be multiplied by 2. The second and third reactions should be reversed. This applies to the corresponding enthalpy values as well. That means, if the reaction is reversed the sign of the corresponding change in enthalpy should also be reversed. The enthalpy of the overall reaction is —988.4 kJ/mol.
E. THE SECOND LAW OF THERMODYNAMICS
We learned how to predict the mode of a reaction in terms of whether the reaction will be endothermic or exothermic. In this section, we will explore the dynamics and the factors that influence the spontaneity of reactions. For that, we have to be familiar with the term “entropy.”
Entropy (S) is the measure of how disordered a system is. The entropy is dependent on the prevailing conditions such as pressure and temperature. From the MCAT point view, the values of entropy and related aspects that you will be dealing with are at standard state. The standard state refers to 1 atm and 25°C.
Entropy of a system is the measure of disorder or randomness of the system.
Let’s talk more about entropy. The higher the disorder is, the greater the entropy of that system. In terms of the phases of matter, think about which phases will have a higher entropy. We can generalize that the gas phase of a substance has higher entropy than its liquid phase, and the liquid phase has higher entropy than its corresponding solid phase. Thus, as the temperature increases the entropy increases. The change in entropy (ΔS) is equal to the final entropy minus the initial entropy.
![]()
According to the second law of thermodynamics, there is an overall natural tendency toward disorder. You have to understand that we are talking about the overall entropy. Do not confuse this with the fact that we sometimes look at individual systems or reactions and categorize them as having either increasing entropy or decreasing entropy. But the overall entropy of all the events together is increasing.
Example 10-2
Predict whether the entropy is increasing or decreasing for the following change.
![]()
Solution:
The question asks whether the entropy of the system is increasing or decreasing for the above written equation. Your response should be that there is an increase in entropy. Why? As we discussed, the change in phase is the key aspect to watch here. Carbon dioxide is changing from its solid form to gaseous form. Hence, the randomness of the system is increasing. So the entropy is increasing.
Example 10-3
Predict whether the entropy of the system is increasing as the reaction given below goes to completion.
![]()
Solution:
The reaction shown above is the combustion reaction of acetylene. This reaction is made use of in welding, commonly called the “acetylene torch.” Tremendous amount of heat is liberated as a result of this reaction and is used for welding purposes. As you can see from the equation, the number of moles of the reactants is greater than the number of moles of the products. Thus, as the reaction proceeds, there is a decrease in the number of moles of gas. If you predicted a decrease in entropy, your answer is correct.
F. THE THIRD LAW OF THERMODYNAMICS
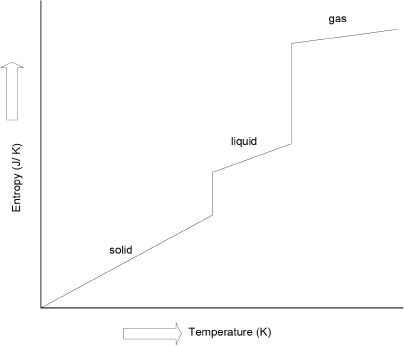
Figure 10-1 Entropy versus temperature
Let’s state the third law of thermodynamics. The law states that pure and perfect crystalline substances have an entropy of zero at 0 Kelvin. What does that mean? It means that a pure crystalline substance will have perfect order at absolute zero temperature. An increase in temperature will destroy this zero entropy. By looking at the graph (Figure 10-1), it is clear that an increase in temperature increases the entropy.
G. FREE ENERGY
Free energy denoted by the letter G is a thermodynamic quantity, with which we can predict the spontaneity of a reaction. The change in free energy (ΔG) is related to the changes in enthalpy and entropy, and temperature as indicated by the equation:
![]()
Here, ΔG represents the change in free energy, ΔH represents the change in enthalpy, ΔS represents the change in entropy, and T represents the temperature.
How Can We Predict Spontaneity?
On the basis of free energy change we can predict the spontaneity of a reaction. You should be able to manipulate the above mentioned equation, and once you have found the free energy change, you can do the prediction using the following guidelines.
If ΔG is positive, the reaction generally is nonspontaneous. If ΔG is negative, then the reaction is generally spontaneous. The changes in free energy and spontaneity depend on the change in enthalpy as well as the change in entropy. Besides these two important factors, the temperature at which the reaction takes place also plays a major role in dictating the spontaneity of reactions. Take a look at Table 10-1.
Table 10-1
ΔG |
ΔH |
ΔS |
Spontaneous at all temperatures |
— |
+ |
Spontaneous at low temperatures |
— |
— |
Spontaneous at high temperatures |
+ |
+ |
Nonspontaneous at all temperatures |
+ |
— |
H. TEMPERATURE — SOME BASICS
We use the term temperature quite a lot in Chemistry, but how can we define it? Students often get confused with the terms temperature and heat. Even though temperature and heat are related, they are not the same. Let us consider two objects that are at different temperatures; a hot object and a cold object. Let’s say we kept them in close proximity for some time. The heat passes from the hot object to the cold one, until they reach equilibrium. So it is the heat (energy) that passes from the hot object to the cold one resulting in a change in temperature.
The common scales used to denote temperature are the Celsius scale and the Kelvin scale (K). The SI unit of temperature is actually kelvin (K). The Celsius and the Kelvin scales can be easily converted back and forth, and are related by the following simple relations:
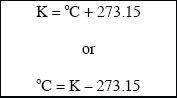
The other temperature scale that we are more familiar with is the Fahrenheit scale. Temperature denoted in degree Celsius can be converted to degree Fahrenheit using the following relation:
![]()
You are expected to know these conversions at this level of study in college. So know them!
CHAPTER 10 PRACTICE QUESTIONS
1. Bomb calorimeter is a device used to determine the energy changes associated with reactions. The calorimeter measures the heat absorbed or evolved during a reaction. Which of the following is true with respect to bomb calorimeter?
A. The measurements are done at constant volume.
B. The measurements are not done at constant volume, because we cannot keep the volume constant.
C. Theoretically work is done during the reaction in a bomb calorimeter.
D. None of the above.
2. Consider the figure shown below in which ice is melted to form water. Which of the following is true regarding this conversion?
A. Entropy is decreased.
B. Entropy is increased.
C. Entropy is not affected since the change does not constitute an actual reaction, but only a change in physical state of H2O.
D. Randomness is decreased.
3. If the change in free energy of a reaction is zero at constant temperature and pressure, what is true regarding such a system?
A. The reaction is spontaneous.
B. The reaction is nonspontaneous.
C. The reaction is at its equilibrium state.
D. Cannot be predicted.
4. Mercury sulfide can be converted to mercury by heating while exposed to air. What is probably true about this reaction?
![]()
A. The reaction is nonspontaneous.
B. The reaction is endothermic.
C. O2 acts as the catalyst.
D. None of the above can be ascertained without further data.
5. All the following are false regarding entropy, except:
A. the entropy of pure substance at 0 K temperature is greater than 1.
B. the entropy of pure substance at 0 K temperature is between 0 & 1.
C. the entropy of pure substance at 0 K temperature is 0.
D. the entropy of pure substance at 0 K temperature is a negative number.