The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Equilibrium and Kinetics
A. INTRODUCTION
Different chemical reactions occur at different rates. Some reactions occur very fast, whereas some others take years and years to complete. A better understanding of chemical kinetics (the study of reaction rates) and equilibrium enables chemists to optimize the production of desirable products. This exploration of chemical kinetics has increased the understanding of biochemical pathways and other pharmaceutical endeavors. We will also discuss catalysts and their effects on reactions.
B. RATE OF REACTION
The rate of a reaction depends on many factors such as the concentration of reactants, the temperature at the time of the reaction, the states of reactants, and catalysts. The rate of a reaction is defined as the change of reactant or product concentration in unit time. If we were to define the rate of a reaction in terms of the reactants, we should define the rate as the rate of disappearance of reactants. If we were to define the rate in terms of the products formed, we should define it as the rate of appearance of products.
For further exploration of this concept, let’s look at a hypothetical reaction. In the hypothetical representative reaction shown below, the small letters denote the coefficients of the corresponding capital letter reactants or products. In this reaction, A and B are reactants, and a and b their coefficients respectively. X and Y are the products, and x and y their coefficients respectively.
![]()
For this reaction, we can represent the rate in terms of the disappearance of each reactant or appearance of each product. The numerators are the concentrations of either the reactant or the product, and Dt represents the elapsed time.
![]()
We can also represent it in terms of reactant B. That looks the same as the rate in terms of concentration of A. In order to express it in terms of B’s concentration, substitute the numerator with the concentration of B. The minus sign convention indicates that the rate is expressed in terms of the rate of disappearance (decreasing concentration) of the reactants.
![]()
Also shown below are the representations of the rate in terms of the products:
![]()
Rate Law
Rate law is an expression that we can find experimentally, which relates the concentration of reactants and the rate of a reaction. Let’s consider the same hypothetical equation:
![]()
For this reaction, we can write the rate as follows:

Note that the rate constant and the exponents of a reaction are found experimentally. For questions related to finding the actual rate expression of a reaction, you will be given the relevant experimental data. We will look at some examples to familiarize ourselves with this concept.
We mentioned the exponent values (for this reaction, we represented them as m and n) in the rate law. Those exponents represent the order with respect to a particular reactant, or by adding all the exponents, you will get overall order of the reaction. For our hypothetical reaction, the reaction order with respect to reactant A is m. The order with respect to reactant B is n. The overall order of the reaction is m+n. That is one way to determine the order of a reaction. Chemists categorize them as first order, second order, etc. You may have heard of zero order. In some reactions, even if a reactant appears in the balanced equation of a reaction, it may not appear in the experimentally found rate expression. The reason for this is that the particular reactant that does not appear in the rate expression has an exponent of 0. So the order with respect to that reactant is zero.
Example 11-1
From the given experimental data, determine the rate law of the hypothetical reaction indicated below:
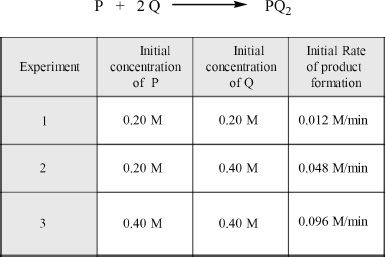
Solution:
The rate of the reaction will have the general look as shown. Assume that the rate law of the reaction is:
Rate = k [ P ]m [ Q ]n
The plan is to find out the actual values of m and n from the given data. Let’s do it step by step.
Look at the given experimental data. If we compare Experiments 1 and 2, we can see that the concentration of Q is doubled in Experiment 2. But the concentration of P is kept constant. With these changes, we see the quadrupling of the rate. That means the exponent of Q is 2. At this point we can rewrite the rate law as follows:
Rate = k [ P ]m [ Q ]2
Now compare Experiments 2 and 3. Here the concentration of Q is kept constant, but the concentration of P is doubled. The rate is doubling because of this change. So the exponent of P is 1. We can now write the completed rate law of the reaction.
Rate = k [ P ] [ Q ]2
C. TRANSITION STATE
From our earlier discussions, we know that exothermic reactions release energy and endothermic reactions consume energy. Here, we will plot the potential energy diagrams of exothermic and endothermic reactions. Before we do that, we will discuss the concept of transition state. Consider the hypothetical reaction shown below:
![]()
Can you say what type of reaction this is? It is an exothermic reaction. For reactions to proceed, there should be breaking of bonds and formation of bonds. For many reactions, the stability barrier that originally made the reactant molecules stable should be overcome. This is achieved by the formation of an energy-state that has higher energy than the reactants and the products. This high-energy state is often referred as transition state. These transition states have a relatively short life span, and once this highly unstable transition state is formed, it quickly disintegrates to form the products. For a reaction to reach this transition energy level, a certain amount of energy is required. This energy is called the activation energy or the energy of activation.
In the diagram (Figure 11-1), the activation energy depicted in both Figures (a) and (b) are for the forward reactions.
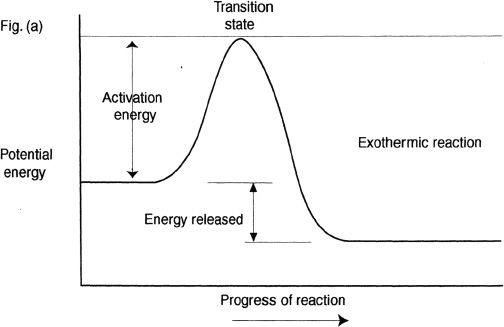
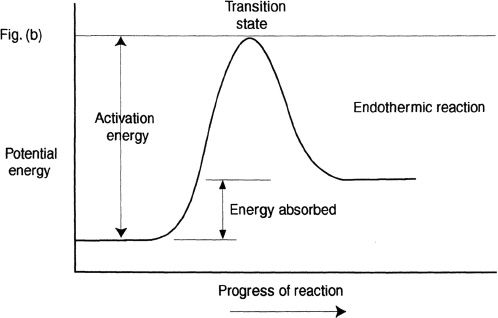
Figure 11-1
D. TEMPERATURE DEPENDANCE OF RATE
The rate of a reaction depends on the prevailing temperature. Hence, the rate law is dependent on the temperature at which the reaction takes place. The reactants must attain a high energy form (transition state) in order for the reaction to occur. For this, the activated reactive species must collide (with feasible orientation) with each other with a minimum kinetic energy which is greater than that of the reactants. This kinetic energy depends on the temperature of the system in which the reaction takes place. At a particular temperature, some of the reactants will attain the activation energy to undergo the reaction. If we increase the temperature, more reactant molecules will attain this energy. More reactants attaining the required energy level means more reaction is taking place.
A mathematical relationship was introduced by Arrhenius. The expression relating the rate constant and the energy of activation is:

There is no need to memorize this equation, but you should understand how to work with the equation conceptually.
E. A BRIEF LOOK AT CATALYSTS
We all have heard of catalysts. In lay terms we know that catalysts speed up reactions. But the question is how do they accomplish this? Consider the formal definition of a catalyst.
A catalyst is a chemical substance that can increase the rate of a reaction. Even if the catalyst is involved in the reaction, by the end of the reaction you will get the catalyst intact. In other words, the catalyst retains its identity. If a substance changes its identity and does not have the original nature after the reaction has occurred, it is not a catalyst, but a reactant.
As we said earlier, the activation energy is the minimum energy required by the reactants to reach the transition state. A catalyst lowers the activation energy of a reaction. Thus it provides a lower energy pathway for the reaction to occur. So the transition state bump in the potential energy diagram (see Figure 11-2) is lowered in a catalyzed reaction, compared to the corresponding uncatalyzed reaction.
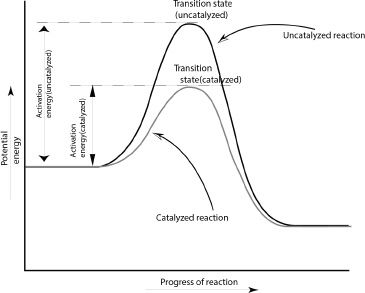
Figure 11-2
Catalysts are often classified on the basis of the phases (solid, liquid, and gas) in which they exist during a chemical reaction. If a catalyst exists in the same phase as the reactants of a reaction, then it is called a homogeneous catalyst. If a catalyst’s phase is different from that of the reactants of a reaction, then it is called a heterogeneous catalyst.
F. CHEMICAL EQUILIBRIUM
Many reactions are reversible. Reversible reactions are reactions in which there are both forward and backward reactions. Consider an experiment in which two reactants have been mixed. At first, the reaction proceeds with considerable rate in the forward direction (forward reaction favored). Before the reaction goes on to completion, the backward reaction takes place. Then again the forward reaction takes place, followed by the backward reaction and so on. These uneven back-and-forth directional changes take place until the reaction mixture reaches the equilibrium. At the equilibrium, the rate will be the same for both forward and backward reactions.
The Equilibrium Constant
We already talked about the rate of a reaction and its general expression. For reactions involving simple one-step mechanisms, we can easily write the rates of forward and backward reactions. Examine a one-step hypothetical reaction represented by the equation (balanced) given below:
![]()
The equilibrium constant of a reversible reaction is equal to the ratio of the product of the concentrations of the products raised to their corresponding coefficients, to the product of the concentrations of the reactants raised to their corresponding coefficients. Keep in mind that the coefficients are taken from the balanced equation. To further clarify this concept, take a look at the mathematical expression for equilibrium constant (Kc) shown below:
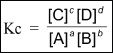
The best way to find out the equilibrium constant of a particular reaction at a given condition is to do it experimentally. Equilibrium constant depends on temperature, and hence it differs with change in temperature. You should also ask yourself this question. Does the initial concentration of the reactants dictate the equilibrium constant? The answer is no.
We can also explore the meaning of equilibrium constant in terms of forward and backward reactions. A small value (less than one) for the equilibrium constant indicates that the forward reaction is not favored. A value greater than one for the equilibrium constant indicates that the forward reaction is favored.
G. FACTORS THAT AFFECT THE EQUILIBRIUM OF REACTIONS
The equilibrium of a reaction is affected by factors such as changes in concentration, pressure, and temperature. The likely resulting changes in equilibrium can be predicted on the basis of Le Chatelier’s principle which can be stated as follows:
If we change the conditions (factors such as the ones mentioned above) of a reaction system in equilibrium, the system will shift in such a way as to reduce the imbalance caused by the stress. This is Le Chatelier’s principle.
Based on this principle, we will look at some of the factors that affect the equilibrium of reactions.
Effect of Temperature
What is your first instinct when you think of a reaction scenario in which the temperature is changed? It is true that the reaction rate will change. We might even think that the reaction rates of all reactions invariably increase. This is not true. Even though many reactions speed up with the increase in temperature, that is not always the case. Consider the reaction given below:
![]()
The reaction between nitrogen and hydrogen to give ammonia is an exothermic reaction. The reaction produces heat energy during the forward reaction. For this reaction at equilibrium, if we increase the temperature, it will only facilitate the backward reaction. Now we will consider another reaction.
![]()
For this reaction, we are supplying heat. So what kind of reaction is it? It is an endothermic reaction. If we subject this reaction in equilibrium to an increase in temperature, the forward reaction will be favored. We can generalize our observations as follows:
An increase in temperature favors endothermic reactions. An increase in temperature decreases the reaction rate, if the reaction is exothermic.
Effect of Pressure
The effects of pressure are significant in reactions involving gases. Think about what happens when we increase the pressure of a reaction in equilibrium. When the pressure is increased, the equilibrium no longer exists. Consider the reaction given below.
![]()
The direction of reactions can be predicted based on Le Chatelier’s principle. For the above reaction, if the pressure is increased the forward reaction is favored. Why is the forward reaction favored? The reason is that the forward reaction will result in less moles of gas, thereby decreasing the strain caused by the increased pressure. This influence of pressure will not affect the reaction if the total number of moles of reactants and the total number of moles of products are equal. The influence of pressure is not seen with reactants or products which are either in their solid or liquid state because they are almost non-compressible.
CHAPTER 11 PRACTICE QUESTIONS
1. Choose the correct equilibrium expression for the following reaction.
![]()
A. ![]()
B. 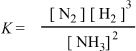
C. ![]()
D. 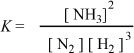
2. Based on the given data, what is the rate law of the following hypothetical reaction?
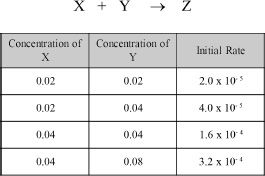
A. Rate = k [ Y ]
B. Rate = k [ X ] [ Y ]
C. Rate = k [ X ]2 [ Y ]
D. Rate = k [ X ] [ Y ]2
3. Which of the following factors will affect the rate of a reaction?
A. The concentration of reactants
B. Temperature
C. The state of reactants
D. All the above
4. Figure 1 represents the potential energy diagram of a reaction. Pick the true statement regarding this reaction.
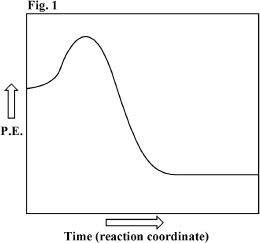
A. The reaction requires no activation energy, since it is spontaneous.
B. There is a net absorption of energy by the reaction.
C. Reactants have lower energy than that of the products.
D. None of the above.
5. Which of the following is true regarding catalysts?
A. Catalysts can change the rate of a reaction.
B. Catalysts increase the activation energy.
C. Catalysts are often consumed completely by the reactants.
D. All the above.
Questions 6-13 are based on the following passage.
Passage 1
At this point of advancements in science, chemists have a good understanding of the kinetics of reactions. They can predict the effects of changes in pH, temperature, and concentration in chemical reactions.
In a cylinder, there is a reaction mixture of hydrogen and iodine. The hydrogen and the iodine react to form hydrogen iodide.
![]()
The industrial production of ammonia is done by a process called Haber process. The reaction occurs as shown below, and is usually done in the presence of an iron catalyst.
![]()
6. If the reverse reaction of Reaction 1 is endothermic, what is the sign of DH of the reaction in which HI is on the product side?
A. Positive
B. Negative
C. Neither positive nor negative, because the reaction is reversible
D. Cannot be predicted, because more data is required regarding the individual enthalpy values
7. Which of the following is most likely to occur, if we take away H2 from the reaction mixture in Reaction 1?
A. The reaction will proceed to the right.
B. The reaction will suddenly reach equilibrium.
C. The reaction will proceed to the left.
D. No apparent effect will be observed.
8. If the pressure applied to Reaction 1 is decreased, what is the most likely effect?
A. The equilibrium will shift toward the left.
B. The equilibrium will shift toward the right.
C. There will be no effect on the equilibrium.
D. The volume of the system will decrease.
9. A student researcher conducted experiments on the effects of changes in the conditions on reactions. The student did the experiments with Reaction 2, by increasing the pressure on the cylinder where the reaction mixture was present. Which of the following are completely true regarding the experiment?
I. The reaction will shift toward the right, because there are more moles on the product side.
II. The reaction will shift toward the left, because there are more moles on the reactant side.
III. The reaction will have increased production of ammonia, because there are more moles on the reactant side.
A. I only
B. II only
C. III only
D. II & III
10. Which of the following best explains the theory behind the predictions of the effects of increasing the concentrations of reactants or products in a reaction system?
A. Dalton’s law
B. Markovnikov’s principle
C. De Broglie principle
D. Le Chatelier’s principle
11. If 70 g of nitrogen is reacted with hydrogen, how many grams of ammonia will be produced? (The weight of hydrogen used = 10 g)
A. 42.5 g
B. 57 g
C. 85 g
D. 17 g
12. In Reaction 2 for the production of ammonia from nitrogen and hydrogen, what is most likely to be true about entropy?
A. Entropy increases and is positive
B. Entropy decreases and is negative
C. Entropy increases and is negative
D. Entropy increases and is positive
13. In the Haber process for the production of ammonia, what can you say about the spontaneity of the reaction with the available information in the passage?
A. The reaction is definitely spontaneous.
B. The reaction is definitely nonspontaneous.
C. The reaction is endothermic, and definitely spontaneous.
D. Cannot be predicted accurately without further data.