The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Electrochemistry
A. INTRODUCTION
Electrochemistry is the study of chemical reactions that result in the production of electric current, and chemical reactions that occur when subjected to electric current. Electrochemical applications are part of everybody’s day-to-day life. Energy storing batteries that we use for TV remotes, flashlights, automobiles are a few examples. Electroplating is another achievement among many others. In this chapter, our discussion will revolve around two types of electrochemical cells - the electrolytic cell and the galvanic cell.
B. ELECTROLYTIC CELL
In an electrolytic cell, electric current drives the chemical reaction. The chemical reaction involved in an electrolytic cell is nonspontaneous. Electric current is used to drive the reaction. This process is called electrolysis and hence the name, electrolytic cell. The reaction involves the transfer of electrons and thus it is a redox reaction. For further understanding of the functioning of an electrolytic cell, we will look at an example of an electrolytic cell involving the electrolysis of molten sodium chloride. Molten sodium chloride is a good conductor of electricity. The melting point of NaCl is around 800° C.
The cell contains molten sodium chloride into which two electrodes are immersed, as shown in Figure 12-1. One electrode is connected to the positive terminal of the battery, and the other is connected to the negative terminal of the battery. The electrode that is connected to the positive terminal of the battery is the anode. The other electrode is the cathode. Reduction occurs at the cathode, and oxidation occurs at the anode. When the current starts flowing the reaction starts, as described below:
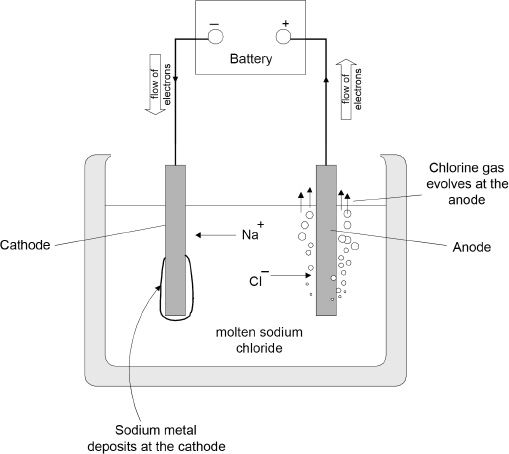
Figure 12-1 Electrolytic cell set up showing the electrolysis of molten NaCl
As the reaction proceeds, the sodium ions (Na+) are reduced to sodium (Na) at the cathode, and the sodium metal is deposited at the cathode. On the other hand, the chloride (Cl—) ions are oxidized at the anode forming chlorine gas (Cl2). The half-reactions and the overall reaction are represented below:
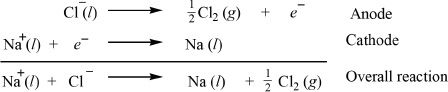
C. FARADAY’S LAW
According to Faraday’s law, the amount of substance that undergoes oxidation-reduction reaction at the electrodes is directly proportional to the amount of electric current that the reaction is subjected to. Faraday constant is equal to the charge of one mol of electrons, and is numerically equal to 96500 coulombs. You probably remember `coulombs’ from your physics undergraduate courses. The unit coulomb is related to the unit ampere (SI unit of current).
![]()
D. GALVANIC CELL
Galvanic cell is also known as voltaic cell. The major difference between an electrolytic cell and a galvanic cell is that the reaction in a galvanic cell is spontaneous, and the reaction produces electric current. The batteries that we use in TV remotes and flash lights are galvanic cells. Galvanic cells convert the stored chemical energy into electrical energy for usage.
A galvanic cell has two half-cells. Each half-cell consists of a metal electrode immersed in a solution containing the same ions. The two half-cells are connected by a wire as shown in Figure 12-2. As we mentioned earlier, the galvanic cell produces electric current. Thus the voltage developed can be measured by setting a voltmeter along the connecting wire, as seen in the figure.
Here, we will look at a cell setup which uses zinc and copper as the electrodes. In addition to the electrodes, the two containers which hold the appropriate solutions and the connecting wire, there is a salt bridge which connects the two solutions. The salt bridge is usually dipped into the solutions of the two half-cells. It contains a gel in which an electrolyte is present. The electrolyte present in the salt bridge will neutralize the buildup of ionic charge in the cells; a buildup which will otherwise slow down and stop the reaction from proceeding.
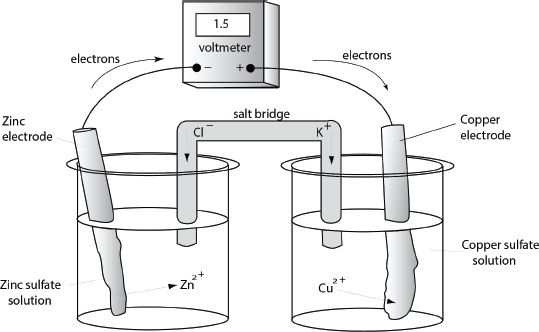
Figure 12-2 Galvanic cell
In the zinc half-cell, a zinc electrode is immersed in zinc sulfate solution. In the copper half-cell, a copper electrode is immersed in copper sulfate solution. The two electrodes are connected by a wire through which there will be flow of electrons resulting from the reaction. The half-reactions are shown below:
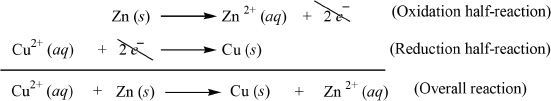
The process of oxidation occurs at the anode and the process of reduction occurs at the cathode. So the first half-reaction (oxidation half-reaction) occurs at the anode, and second half-reaction (reduction half-reaction) occurs at the cathode. The overall reaction can be obtained by adding the two half reactions. Here, the zinc electrode is the anode, and the copper electrode is the cathode. In a galvanic cell, the anode is the negative electrode and the cathode is the positive electrode. As far as electron flow is concerned, the flow is always from the anode to the cathode.
Let’s take a look at Figure 12-2 again. Notice the irregular edges of the electrodes. Why is that so? The reason is simple. As the reaction proceeds, zinc is stripped away from the zinc electrode, and thus it becomes thinner and thinner until the reaction stops (when it is at equilibrium). On the other hand, the copper electrode becomes thicker and thicker due to the deposition of metal copper on the copper electrode.
The half-reactions are often represented by the notation shown below. By convention, the oxidation reaction is written on the left of the symbol denoting the salt bridge, and the reduction reaction is written on the right side of the salt bridge symbol.
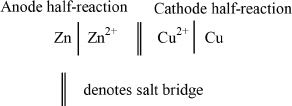
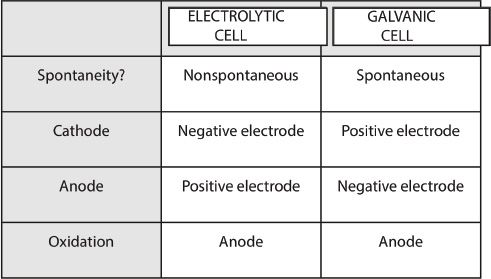
Note: See Table 12-2 at the end of this chapter for electrolytic versus galvanic cell comparison.
E. STANDARD ELECTRODE POTENTIALS
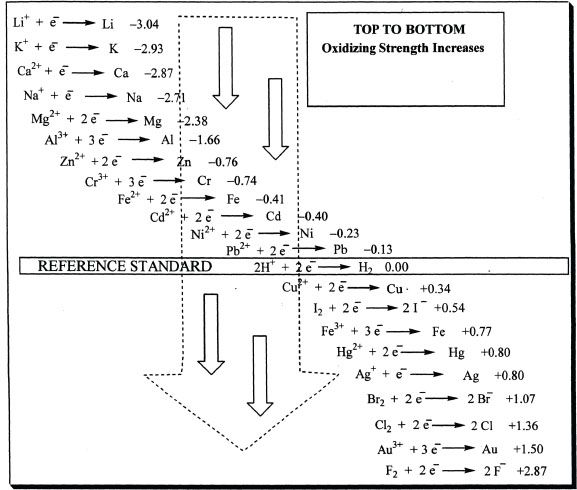
Before we discuss standard electrode potential, we will talk about electromotive force (emf). The electromotive force of a cell is the potential difference between the two electrodes. This can be measured using a voltmeter. The maximum voltage of a cell can be calculated using experimentally determined values called standard electrode potentials. By convention, the standard electrode potentials are usually represented in terms of reduction half-reactions. The standard electrode potential values are set under ideal and standard-state conditions (1atm pressure and 25°C temperature). From the MCAT point of view, you can assume that the conditions are standard, unless stated otherwise. Table 12-1 shows a list of standard electrode potentials (in aqueous solution) at 25°C.
The standard electrode potential at the above mentioned standard state conditions is denoted by E °. For the MCAT, the values of the standard electrode (reduction) potentials will be given to you if you are required to solve such a question. Do not try to memorize those values. The standard electrode potentials are based on an arbitration with reference to standard hydrogen electrode. The standard hydrogen electrode potential is considered to be 0 volt.
Table 12-1 Some Standard Electrode (Reduction) Potentials
Finding the emf of a Cell
The emf of a cell can be calculated from the standard electrode potentials of the half-reactions. In order to find the emf, we have to look at the two half-reactions involved in the reaction. Then, set up the two half-reactions so that when they are added we will get the net reaction. Once we have set the equations properly and assigned the proper potentials to those half-reactions, we can add the standard electrode potentials. A common mistake that students make is that they forget the fact that the standard electrode potentials are given in terms of reduction reactions. Redox reactions involve both oxidation and reduction. If one half-reaction is reduction, the other should be oxidation. So we must be careful about the signs of the half-reaction potentials, before we add the two half-reaction potentials to get the emf value. Do the next example.
Example 12-1
Calculate the emf of the cell, based on the following net reaction.
![]()
Standard Electrode Potentials of the half-cells
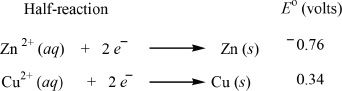
Solution:
First, we have to write the half-reactions as indicated below.
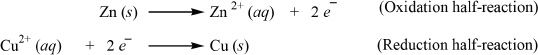
From Table 12-1, we can take the standard electrode potential values. The cell containing the copper electrode has a standard potential value of 0.34 V. For the other half-cell, the reaction is oxidation. Since the value given in the table is in terms of reduction half-reactions, we have to reverse the sign of the standard electrode potential given. The correct value for the oxidation half-cell is +0.76 V instead of —0.76 V. Now, you can add the two values to get the emf of the whole setup.
emf = 0.34 + 0.76 = 1.10 V
The answer is 1.10 V.
Table 12-2 Electrolytic cell & Galvanic cell - A comparison
F. THE FREE ENERGY-EMF RELATION
The change in free energy (ΔG) is the maximum amount of energy that is available to do useful work. In an electrochemical cell, this free energy is equal to electrical work which is equal to the product of the number electrons, the Faraday constant, and the electrochemical cell’s emf.
![]()
In this equation, n is the number of equivalents of electrons transferred in the reaction, F is the Faraday constant (96,500 Coulombs), and E0cell (cell’s emf). From this equation, we can deduce that if the emf is positive, the corresponding change in free energy (ΔG) will be negative. In other words, if the emf is positive the reaction is most likely to be spontaneous. On the other had, if the emf of a cell is negative, the ΔG will be positive indicating a nonspontaneous reaction.
Example 12-2
Calculate the standard free energy change at 25°C for the redox reaction in Example 12-1. (Faraday constant = 96,500 coulombs)
Solution:
![]()
From the previous example, we know that the emf of this reaction is 1.10 V. The formula for ΔG in terms of the potential difference is:
![]()
Here, the number of electrons transferred is 2. This number is obtained by examining the balanced equation and evaluating the change in oxidation numbers. For example, copper ions with +2 oxidation state changed to copper (solid) with an oxidation state of 0. In other words, each half-reaction involves two electrons
![]()
Notice that the change in free energy is negative and this indicates that the reaction is likely to be spontaneous.
CHAPTER 12 PRACTICE QUESTIONS
1. Given below is the standard electrode potential (E°) of the following redox reaction. Predict the most feasible event, if the reaction occurred spontaneously.
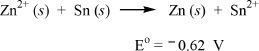
A. Zn2+ was reduced.
B. Sn was oxidized.
C. Zn lost electrons.
D. Ether was used as the solvent medium.
2. What is the standard electrode potential of Reaction 1 given that:
![]()
A. —0.13 V
B. +0.13 V
C. +0.26 V
D. +0.52 V
3. The standard potential E° is related to equilibrium constant K by the following relation:
—nFE° = —RT ln K
In a reaction occurring at standard state conditions, if the concentrations of the products are greater than that of the reactants, which of the following is true?
A. K is negative.
B. E° is negative.
C. The reaction is nonspontaneous.
D. None of the above
4. In an electrolytic cell, 5 A of current passes for about 3.5 minutes. The amount of electric charge equals:
A. 17.5 coulombs.
B. 85.7 coulombs.
C. 1050 coulombs.
D. 96500 x 5 x 3.5 coulombs.
Questions 5-10 are based on the following passage.
Passage 1
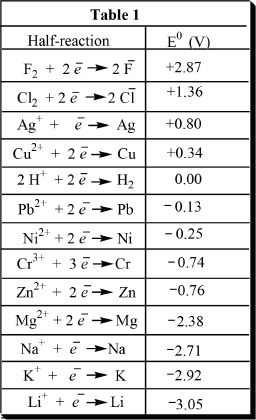
By comparing standard reduction potentials, we can compare the reduction and oxidation powers of elements or ionic species. Some standard reduction potential values are given in Table 1.
5. Which of the following processes is most likely to be a spontaneous process?
A. Oxidation of copper
B. Reduction of chlorine
C. Oxidation of fluoride ion
D. Reduction of zinc ion
6. What is the overall cell potential for a cell which is formed with Ni/Ni2+ and Ag/Ag+ half-cells?
A. +1.85 V
B. +0.55 V
C. —1.85 V
D. +1.05 V
7. In a galvanic cell, usually a salt bridge is used. What is the most likely purpose of this?
A. To conduct electricity
B. To prevent corrosion
C. To regulate electricity
D. To maintain neutrality in the half-cells
8. Which of the following will act as an oxidizing agent the most?
A. Na+
B. Ag+
C. Cl2
D. Pb2+
9. Which of the following species has the highest oxidation potential?
A. Cr
B. K
C. Cu
D. Ag
10. The process of corrosion is a redox reaction. Consider the rusting of the body panel of automobiles. Certain parts of the object that are undergoing corrosion act as if they were half cells. For the corrosion of iron, iron is changed to Fe2+ ions. Which of the following best represents the area where this change occurs?
A. Cathode
B. Anode
C. Both anode and cathode
D. Cannot be predicted without more data