The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Radioactivity
A. INTRODUCTION
This is the last chapter in Part I of the general chemistry review. In this chapter, we will discuss the different aspects of radioactivity. Radioactivity is a nuclear phenomenon. It results from natural nuclear instability or externally induced nuclear instability. We will limit our discussion of nuclear chemistry to the basic aspects of radioactivity involving radioactive emissions such as alpha emission, beta emission, gamma rays, positron emission, and electron capture. We will also review other ideas such as the half-lives of radioactive substances and the mass-energy equation.
B. STABILITY OF NUCLEUS
The nucleus is not involved in normal chemical reactions. In radioactivity, it is the nucleus that is undergoes the changes. Nuclear forces are extremely strong forces that hold the nuclear particles together. These forces make the nucleus very stable. Remember that the protons are positively charged and can exert repulsive forces among themselves. It has been found out that there are certain predictable behavioral patterns in elements as the atomic number increases. As the atomic number increases, the neutron-to-proton ratio increases and as more and more protons are present in the nucleus, the stabilizing forces are not sufficient to keep the nucleus stable. Thus the stability of the nucleus decreases as the neutron-to-proton ratio increases.
C. RADIOACTIVE DECAY
Atoms with unstable nuclei can undergo radioactive decay to become atoms which are more stable than their parent atoms. In the process, different types of particles are emitted. We will discuss some of the important ones that you have to know from the MCAT point of view.
Alpha emission:
Alpha emission (a) is a low-penetrating emission. It is actually helium nucleus and is often represented as
![]()
An example of radioactive decay of radium-226 is given below:
![]()
As you can see, the resulting atom has both mass number and atomic number changed. The atomic number decreases by 2, and the mass number decreases by 4.
Beta emission:
Beta particles (β—) are emissions having medium level penetration. They are fast traveling electrons. As a result of beta emission, the resulting atom will have an increase in the atomic number by 1. There is no change in the mass number. In the process, there is also a proton formation from the neutron inside the nucleus, along with the electron formation. In the following example, thorium-234 decays to protactinium-234 by emitting a beta particle.
![]()
Positron emission:
Positron emission (β+) is the positive counterpart of an electron emission. A positron has the exact mass of an electron, but has a positive charge. During this event, a proton is converted to a neutron and a positron. The product of a positron decay will have an atomic number less than that of the decayed atom by one unit. There is no change in mass number.
Electron capture:
As a result of electron capture, a proton is converted into a neutron. The electron is usually captured from the innermost shell of the atom. The atomic number of the product will be one less than that of the original atom. There is no change in mass number.
Gamma emission:
Gamma (γ) emissions or gamma rays, as they are commonly referred to, are highly penetrating and dangerous emissions. They are high frequency electromagnetic rays. Gamma rays travel at the speed of light. The resulting product atom has the same atomic and mass numbers as those of the parent atom from which the gamma rays are emitted. Gamma rays have no charge.
D. HALF-LIFE
All radioactive elements do not decay at the same pace. They have drastically different rates of decay. The radioactive decay time is expressed in terms of half-life period. The half-life of a radioactive substance is the time required for the decay of half the substance present in a sample of that substance. Regardless of the amount of a particular radioactive substance we have, it takes the same time (half-life) to complete the decay of half the number of nuclei in that sample.
The half-life of a radioactive substance is the time required for the complete decay of exactly half the amount of that substance.
Example 13-1
Calculate the amount of time (in years) it takes for the decay of 75% of a given sample of carbon-14. Carbon-14 has a half-life of approximately 5700 years.
Solution:
After the first 5700 years of decay, 50% of the original sample is left. After 5700 more years, 50% of that sample will have decayed, which means that there is now 25% of the original intact sample. This is the amount of time that the question is asking for. To be clear about our analysis, let’s rephrase what we have said. We have 25% of the original sample left at this point. Thus the decay of 75% of the original sample is complete. So the answer is 5700 x 2 = 11400 years.
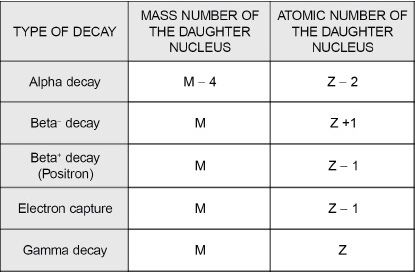
Table 13-1 Summary of changes in the parent nucleus due to different decay modes.
M = mass number of the parent nucleus undergoing decay.
Z = atomic number of the parent nucleus undergoing decay.
CHAPTER 13 PRACTICE QUESTIONS
1. Radium 226 can undergo radioactive decay to form Radon 222. Which of the following is the most likely type of particle that is emitted?
A. Beta particle
B. Alpha particle
C. Gamma particle
D. Positron particle
2. Consider the radioactive-decay equation given below. What is the most likely identity of X?
![]()
A. Alpha particle
B. Beta particle
C. Positron particle
D. Neutron
3. Which of the following emissions travels at the speed of light?
A. Gamma ray
B. Beta particle
C. Alpha particle
D. Antineutrino
4. Which of the following has the highest penetrating power?
A. Alpha particles
B. Beta particles
C. Gamma rays
D. All the above have the same penetrating power
5. Substance X has a radioactive half-life of 12 years. How much time must have elapsed if only 9 grams is left from an original sample of 150 grams?
A. 12 years
B. 24 years
C. 36 years
D. 48 years
Questions 6-11 are based on the following passage.
Passage 1
In nuclear reactions, significant changes occur in the composition of the nuclei of the atoms involved. These reactions usually release tremendous amounts of energy. One of the reasons for the nuclear changes can be attributed to the stability of the nucleus.
The formation of nucleus from the subatomic particles - neutrons and protons, results in the release of energy. The mass of these individual particles in the nucleus is greater than that of the actual nucleus that is formed. This loss of mass is due to the change of mass into energy. The energy-mass relation can be represented in terms of the equation:
E = mc2,
where m represents the mass, and c represents the speed of light (3x108 m/s). If the nucleus of an atom is not stable, it can get transformed into another nucleus. A plot of the number of neutrons versus the number of protons is often used to assess the stability trends of elements. If the number of protons and neutrons are equal, the nucleus is considered to be reasonably stable. As the atomic number increases, the trend changes.
Isotopes of elements having atomic numbers above ≈83 are unstable atoms. These unstable atoms can undergo disintegrations. The half-lives of some radioactive elements are shown in Table 1.
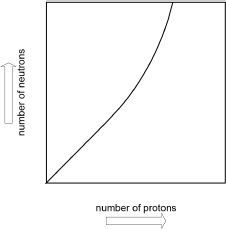
TABLE 1
atom |
half-life |
Carbon 14 |
5.73 × 103 years |
Uranium 238 |
4.47 × 109 years |
Radon 222 |
3.82 days |
Radium 226 |
1.6 × 103 years |
Krypton 89 |
3.2 minutes |
6. A sample of 226Ra disintegrates to 3% of its original quantity. How many half-lives have this sample passed?
A. two
B. three
C. four
D. five
7. 234Th undergoes radioactive decay. If the thorium nucleus emitted a β-particle during its decay, what is the identity of the element that is formed?
A. 234Pa
B. 234Ac
C. 234Ra
D. 232U
8. All the following are true regarding radioactive rays, except:
A. α-particles are positively charged.
B. β-particles are negatively charged.
C. γ rays are electromagnetic rays and can be deflected by an electric field.
D. There are radioactive emissions in which the mass number is not affected.
9. Which of the following is true regarding radioactivity?
A. As the atomic number increases, eventually the neutron-proton ratio values become ≤1.
B. As the atomic number increases, eventually the neutron-proton ratio values become ≥1.
C. As the atomic number increases, eventually the proton-neutron ratio values become ≥1.
D. None of the above
10. When a helium nucleus is formed, there is always some degree of loss of mass. If the loss of mass equals 3.1 x 10—5 kg during the formation of one mol of it, what is the binding energy?
A. 3.1 x 10—5 J
B. 1.8 x 1019 J
C. 9.3 x 103 J
D. 2.8 x 1012 J
11. The most probable set of particles that were given off during the series of nuclear changes from 232Th to 224Ra are:
A. two alpha particles and one beta particle.
B. one alpha particle and two beta particle.
C. one alpha particle and three beta particles.
D. two alpha particles and two beta particles.