The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
General Concepts
A. INTRODUCTION
Organic Chemistry is the study of carbon compounds. Carbon can form a wide array of compounds, because of its size and ability to form covalent bonds with other carbon atoms. In addition, carbon can form bonds with many other elements. This property of carbon increases the facility of forming multitudes of different compounds. The particular electronegativity of carbon also plays a key role in its versatility. In this chapter, we will review some of the fundamental aspects of carbon atom and the main types of hybridizations involving carbon compounds.
B. THE CARBON ATOM
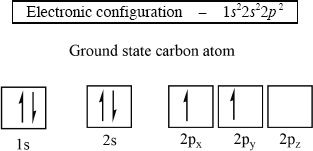
Electrons are found in regions around the nucleus in an atom, and those regions are called orbitals. The orbitals can be defined and differentiated by size, shape, and orientation. Valence electrons are electrons that are found in the outermost shell. The carbon atom has four valence electrons. These valence electrons are involved in chemical reactions and bonding.
The electronic configuration:

C. BONDING
Ionic bond — Ionic bond is formed between an electropositive and electronegative atom (ion), or generally we can define it as an attractive force between a positive and a negative ion (e.g., KCl).
Covalent bond — Covalent bond is formed by the sharing of a pair of electrons between two atoms. Carbon compounds generally contain covalent bonds.
For a more detailed discussion of this topic see the general chemistry section of this book — Chapter 5.
D. HYBRIDIZATION OF ORBITALS
The six electrons of a carbon atom are distributed in the orbitals as follows:
sp3 Hybridization
The carbon atoms of alkanes (e.g., methane, ethane) are sp3 hybridized. In order to form the four bonds in methane, a carbon atom needs four half-filled orbitals. In order to have more free half-filled orbitals, the carbon atoms undergo hybridization.
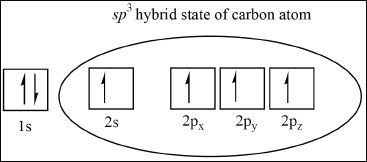
The hybridization results in one half-filled 2s orbital, and three half-filled 2p orbitals (a total of four half-filled orbitals). These unpaired electrons form the sp3 hybridized carbon, which can form the four covalent bonds in the methane molecule. The four sp3 hybrids are directed to the corners of a tetrahedron with bond angles of 109.50.
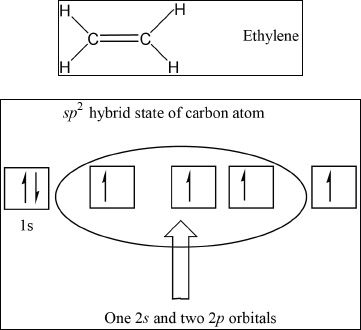
sp2 Hybridization
In carbon-carbon double bonds, the carbons undergo another type of hybridization called the sp2 hybridization. In this hybridization, only one 2s, and two 2p orbitals are involved. The C=C contains a sigma (σ) bond and a pi (π) bond. The pi bond is formed by the unhybridized 2p orbital overlap. The three equal hybrids lie in an xy-plane with bond angles of 1200.
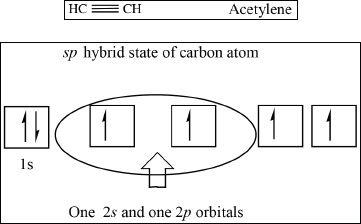
sp Hybridization
Yet another hybridization called the sp hybridization exists in carbon-carbon triple bonds. An sp hybridized carbon atom is bonded only to two other atoms. In this type of hybridization, one 2s orbital and one 2p orbital are involved. A carbon-carbon triple bond contains one sigma bond and two pi bonds.
E. RESONANCE
Resonance is an important aspect in Organic chemistry. Though we represent definite Lewis structures of molecules, in reality the electrons are not localized. They are shared and delocalized by the atoms in a molecule to have the most stable electron distribution. This is called resonance. Resonance promotes stability.

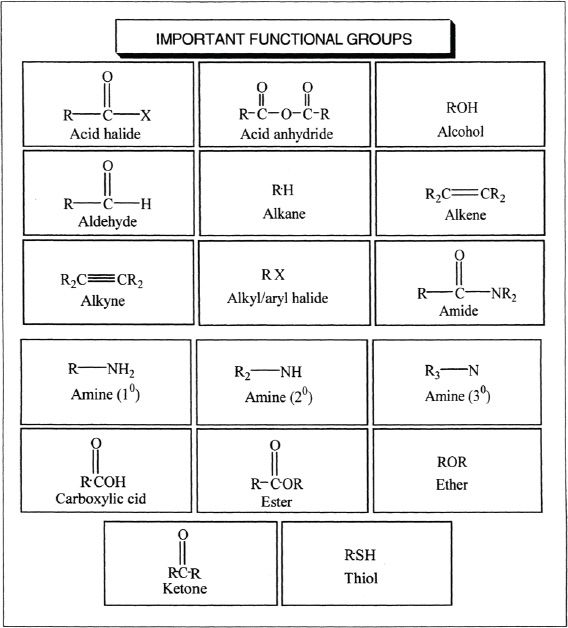
F. FUNCTIONAL GROUPS
CHAPTER 1 PRACTICE QUESTIONS
1. The carbon indicated by the arrow has which of the following hybridizations?
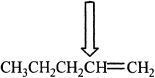
A. sp hybridization
B. sp2 hybridization
C. sp3 hybridization
D. sp3d2 hybridization
2. In acetylene, how many sigma bonds are present between the two carbons?
A. One
B. Two
C. Three
D. Four
3. The triple bond of 2-propyne contains:
A. one sigma bond and one pi bond.
B. one sigma bond and two pi bonds.
C. two sigma bonds and one pi bond.
D. three sigma bonds.
4. Which of the following represents the arrangement of the sp3 hybrid orbitals in methane?
A. Planar
B. Tetragonal planar
C. Tetrahedral
D. Bipyramidal
5. How many electrons are actually involved in the carbon-carbon bond of acetylene?
A. 2
B. 3
C. 4
D. 6
6. Which of the following best represents the hydrogen-carbon-hydrogen bond angles in methane?
A. 90°
B. 109.5°
C. 120°
D. 180°
7. Which of the following bonds indicated by the arrows has the shortest bond length?
A. a
B. b
C. c
D. d
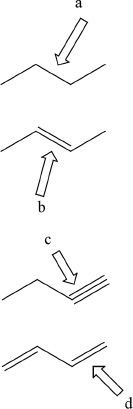
Figure for Q-7
8. The carbon-hydrogen bond in propane can be best described as:
A. an ionic bond.
B. a covalent bond.
C. a hydrogen bond.
D. a dipole-dipole bond.