The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Alkanes
A. INTRODUCTION
Hydrocarbons are compounds containing only carbon and hydrogen. They are classified into aliphatic and aromatic hydrocarbons. Aliphatic hydrocarbons can be divided into three major groups — alkanes, alkenes, and alkynes. Alkanes come under saturated hydrocarbons, because they have carbon-carbon single bonds. Alkenes and alkynes are unsaturated hydrocarbons, since they have carbon-carbon double and triple bonds, respectively.
B. ALKANES
Alkanes have the general molecular formula CnH2n+2. Hence, if we know the number of carbons present in an alkane, we can calculate the number of hydrogens in it or vice versa. Methane is the first member of the alkane family. It has a molecular formula of CH4. Natural gases which are found in petroleum deposits contain gases such as methane, ethane, and propane. They are the first three members of the alkane family.
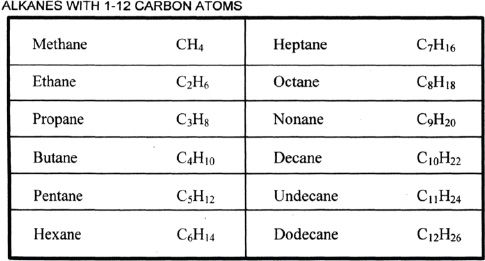
Methane is a colorless, odorless gas. Ethane, propane, and butane are also gases, with butane having the highest boiling point among these. What kind of trend can we see from these observations? As the number of carbons and hydrogens increases, the boiling point increases. Each carbon in an alkane is sp3 hybridized.
C. PROPERTIES OF ALKANES
At room temperature, the first four members of the alkane family are gases. The straight chain alkanes from pentane and up are liquids, and octadecane (18 carbons) and up in the alkane family are solids. As the number of carbons increases, the boiling point increases. Branched alkanes have lesser boiling points than their unbranched or less branched isomeric counterparts. The reason for this is that the unbranched molecules have more intermolecular interactions than the branched ones.
D. STRAIGHT CHAIN AND BRANCHED ALKANES
Butane (C4H10) and the other alkanes above it can exhibit constitutional isomerism. If the alkane is unbranched and has a straight chain, it is called n-alkane. For example, the straight chain pentane is called n-Pentane.
n-Pentane CH3CH2CH2CH2CH3
n-Butane CH3CH2CH2CH3
Constitutional (structural) isomers are isomers with the same molecular formula, but are different in terms of the order in which the atoms are connected.
Butane has two possible isomers: n-Butane and Isobutane
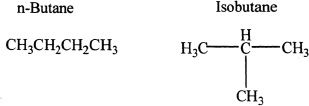
Pentane has three isomers: n-Pentane, Isopentane, and neopentane
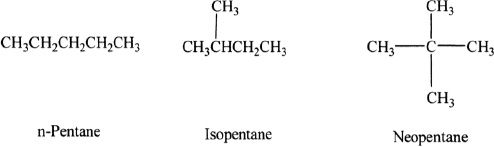
The only way to find the number of possible isomers is by drawing out the structures sequentially and systematically, starting from the straight chain compound. There is no simple general formula to calculate the number of possible isomers of an alkane.
E. ALKYL GROUPS
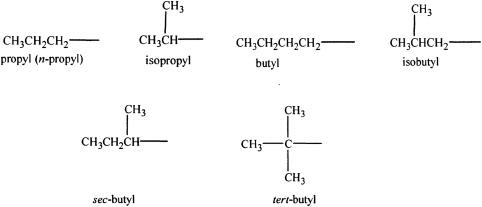
Alkyl groups are groups which lack one hydrogen atom compared to its parent alkane. For example, methyl group is CH3—, which lacks one hydrogen atom with respect to its parent alkane, methane (CH4). Similarly, ethyl group (C2H5—) lacks one hydrogen atom compared to ethane (C2H6).
Some common alkyl groups
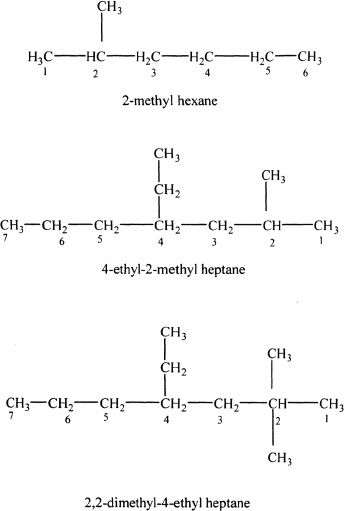
F. THE IUPAC NAMING OF ALKANES
Main rules and strategies for the IUPAC naming of alkanes
1. Write out the expanded structural formula, if it is not given in the expanded form.
2. Find the longest carbon chain.
3. Then identify the alkyl or other substituents that are connected to this long chain.
4. The numbering of carbons should start from the specific end of the long chain, so that the numbers assigned for the substituents are the lowest.
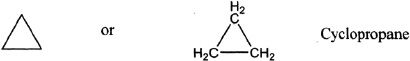
G. CYCLOALKANES
Cycloalkanes are cyclic compounds with ring structures. The general molecular formula of a cycloalkane is CnH2n.
H. REACTIONS OF ALKANES
Combustion
Hydrocarbons undergo combustion reactions in the presence of oxygen to form carbon dioxide and water as products. Combustion reactions are very exothermic giving out energy, as they burn in the presence of oxygen.
Sample reaction 15-1
![]()
Halogenation
The halogenation reaction can be generalized as follows:
![]()
In this substitution reaction, the halogen (fluorine, chlorine, bromine, iodine) substitutes one hydrogen atom in the alkane, forming hydrogen halide and alkyl halide as the products. The reactivity of halogens in the halogenation reactions is as follows:
![]()
Fluorine is the most reactive among halogens in halogenation reactions, and iodine is the least reactive.
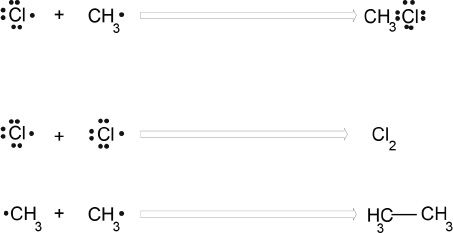
I. MECHANISM OF FREE RADICAL SUBSTITUTION OF ALKANES
Halogenation reactions occur via a mechanism called free radical substitution. There are three main steps in the free radical mechanism. They are:
(1) initiation
(2) propagation
(3) termination.
The overall reaction of chlorination of methane.
![]()
(1) Initiation
This step involves the dissociation of the halogen molecule (e.g., chlorine molecule) into two chlorine atoms. Even though the total reaction is exothermic, initially energy should be supplied for the reaction to proceed.

(2) Propagation steps
During the propagation step, the hydrogen atom is abstracted from methane by a chlorine atom. This is followed by the reaction between the methyl radical and the chlorine molecule.


(3) Termination
The termination steps involve the combination of the radicals.
J. REACTIVITY OF ALKANES
Primary, Secondary, and Tertiary Carbons
A carbon which is attached directly to only one other carbon is called a primary (10) carbon. If it is attached directly to two other carbons, it is a secondary (20) carbon. A carbon is called a tertiary (30) carbon, if it is directly attached to three other carbons.
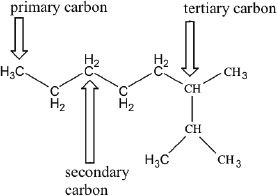
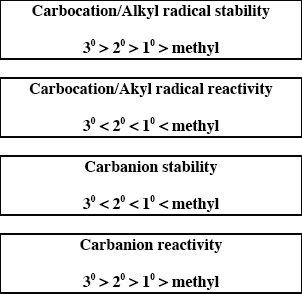
Though alkanes are not so reactive, they can undergo some reactions by forming intermediates. These intermediates can be alkyl radicals, carbocations, or carbanions. Alkyl radicals are intermediates of free radical reactions. Carbocations (carbonium ions) are species with a positive charge on one of the carbon atoms. A carbanion has a negative charge on one of its carbon atoms. Some major trends are given below:
K. CONFORMATION AND STABILITY OF ALKANES
Conformations
Alkanes can have different conformations. By analyzing the structure of ethane, we can define certain aspects regarding its conformations. Conformations are different arrangements of the atoms in a molecule, as a result of rotation around a single bond.
Figure 15-1
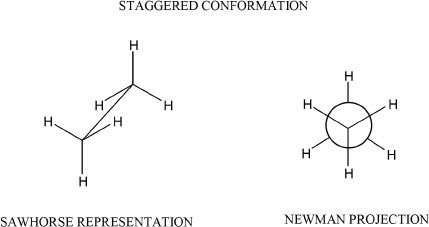
In staggered conformation, the torsional angle is 600. In eclipsed conformation, each carbon-hydrogen bond is aligned with the carbon-hydrogen bond of the next carbon.
Figure 15-2
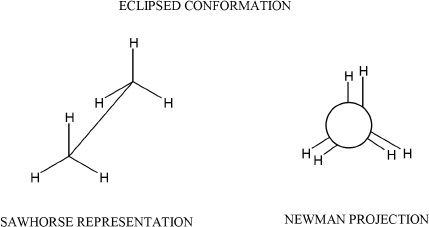
In eclipsed conformations, the torsional angle is 0°. In staggered conformations, the torsional angles can either be 60° (gauche) or 180° (anti). The anti conformation is more stable than the gauche conformation. We should also consider the fact that in this analysis of staggered conformation, the ethane molecule looks the same in the Newman projections, whether it is gauche or anti. Reason: There are no substituents other than just hydrogens. To denote the positional significance, the hydrogens are indicated in bold in the diagrams shown in Figure 15-4.
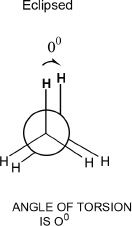
Figure 15-3
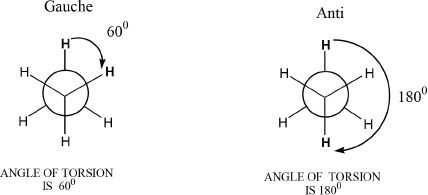
Figure 15-4
L. CONFORMATION AND STABILITY OF CYCLOALKANES
According to Baeyer strain theory, the stability of a cycloalkane is based on how close its angles are to 109.5°. The closer the angle is to 109.5°, the more stable the cycloalkane. Deviation from this angle can cause angle strain. An increase in the angle strain means a decrease in the stability of the molecule.
Conformations of Cyclohexane
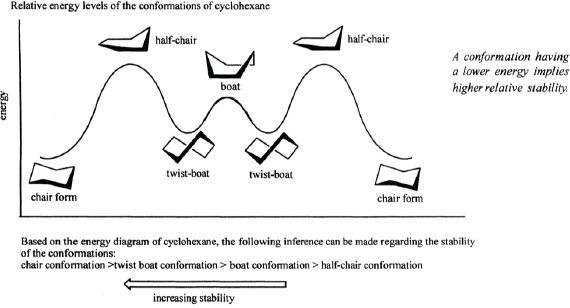
Cyclohexane has a non-planar structure that makes it almost free from ring strain. The most important conformations that it can have include chair conformation and boat conformation. The chair conformation is more stable than the boat conformation. The boat conformation can sometimes be more stable than it usually is, by a slight rotation in the C-C bonds and is called the skew boat conformation. Nevertheless, the chair conformation is the most stable cyclohexane form.
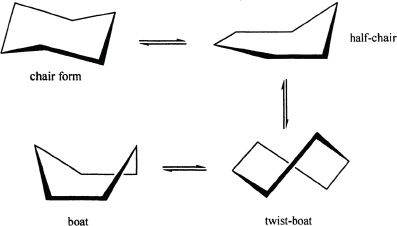
Figure 15-5
In the chair form of cyclohexane, there are two different kinds of carbon-hydrogen bonds. Of the twelve carbon hydrogen bonds in a cyclohexane, six bonds are pointed up or down and are called axial bonds. The remaining six carbon-hydrogen bonds are pointed at an angle out of the ring and are called equatorial bonds. Each carbon atom in the ring is attached to one hydrogen atom by an axial bond, and to the other hydrogen atom by an equatorial bond.
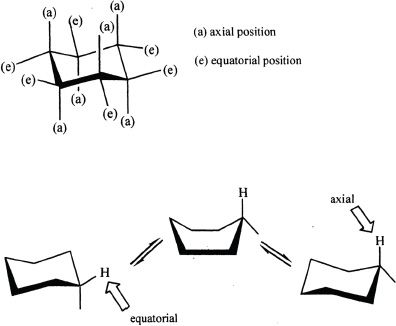
When ring flipping occurs between conformers, equatorial groups become axial, and axial groups become equatorial.
Experimental analysis has confirmed that among the two chair conformations of methylcyclohexane, 95% of the molecules have their methyl group in the equatorial position. In other words, the methyl group being in the equatorial position is more stable than methyl group in the axial position. The lower stability of the axial position can be attributed to the fact that there is an increased steric hindrance because of the proximity of the axial methyl group to the axial hydrogens that are attached to carbon atoms 3 and 5. This interaction called 1,3-diaxial interaction results in steric strain. This accounts for the increased relative stability of the conformation when the methyl group is in the equatorial position.
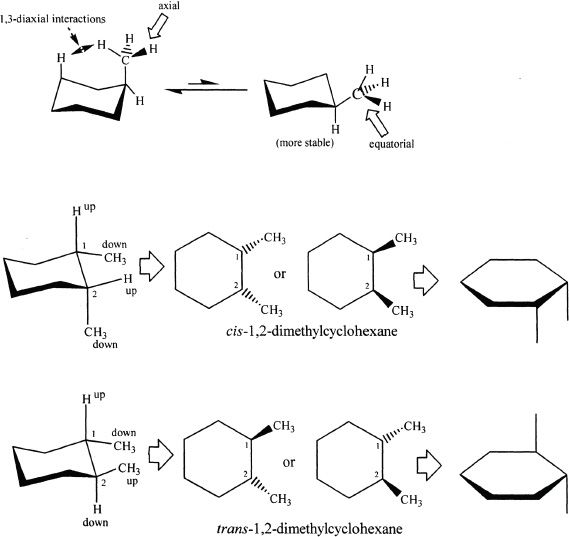
Example 15-1
The cis-geometric isomer of 1,3-dimethylclohexane is more stable than its trans-isomer. Why?
Solution:
To understand why the cis-geometric is more stable, let’s draw the possible chair forms of 1,3-dimethylclohexane.
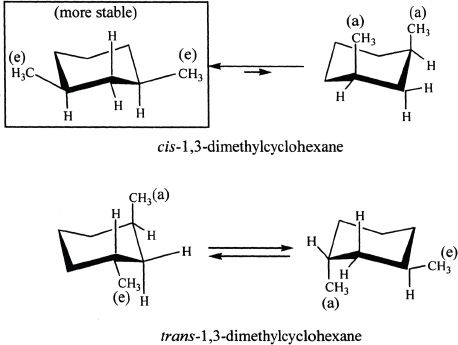
When the two methyl substituents are in 1,3 positions, the cis-isomer can have both substituents in equatorial position. In the trans-isomer, one methyl group must always be axial.
CHAPTER 15 PRACTICE QUESTIONS
1. A student is assigned to identify an unknown compound in an organic chemistry class. She is sure that the compound contains only carbon and hydrogen atoms. In addition, the unknown compound is a saturated hydrocarbon. If there are fourteen hydrogens in a molecule of this unknown compound, and it does not have a ring structure, what is the most likely number of carbons in this compound?
A. 3
B. 6
C. 7
D. 14
2. Which of the following represents the general formula of an alkane?
A. CnH2n+2
B. CnH2n
C. CnH2n—2
D. CnHn
3. n-Butane and isobutane are best described as:
A. stereoisomers.
B. anomers.
C. diastereomers.
D. constitutional isomers.
4. Choose the correct name of the following compound from the choices given below.
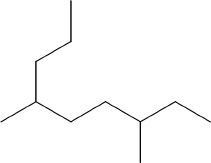
A. 2-propyl-5-methyl heptane
B. 3-methyl-6-propyl heptane
C. 3,6-dimethyl nonane
D. 4,7-dimethyl nonane
5. In the combustion reaction of butane, how many moles of carbon dioxide are formed, if one mole of butane undergoes complete combustion in a controlled environment in the presence of excess oxygen?
A. one
B. two
C. four
D. eight
6. Which of the following represents secondary carbons?
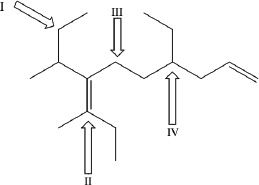
A. I & II only
B. I & III only
C. II & IV only
D. I, II, III & IV
7. Carbonium ions have:
A. a positive charge.
B. a negative charge.
C. no charge.
D. either a positive or a negative charge.
8. The gauche conformation is a form of:
A. eclipsed conformation.
B. anti-conformation.
C. staggered conformation.
D. none of the above conformations.
9. In cycloalkanes which of the following bond angles will have the least angle strain?
A. 90°
B. 110°
C. 125°
D. 180°
10. Which of the following alkanes has the highest boiling point?
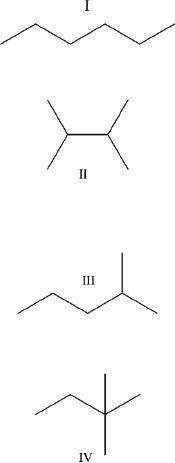
A. I
B. II
C. III
D. IV
Questions 11-15 are based on the following passage.
Passage 1
Hydrocarbons are compounds composed of carbon and hydrogen atoms. Alkanes are hydrocarbons. As the number of carbons increases in straight-chain alkanes, there is a steady gradation of properties which can be easily compared and predicted. The properties of branched alkanes vary considerably and are hard to predict because of other intervening forces that come into play. The boiling points of a few alkanes are given below:
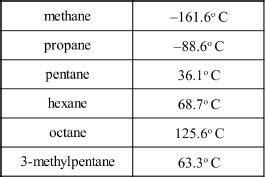
11. Which of the following intermolecular forces are important with respect to alkanes?
A. Hydrogen bonding
B. Dipole-dipole electrostatic forces
C. Ionic forces
D. Van der Waals forces
12. The melting point of butane is close to:
A. 37.5° C.
B. 55.1° C.
C. 24°C.
D. —138° C.
13. Alkenes can undergo free radical substitution reactions with halogens. Which of the following best represents a chain propagation step during the free radical chlorination of methane?
A. ![]()
B. ![]()
C. ![]()
D. ![]()
14. What is the most likely boiling point of 2,3-dimethylbutane?
A. 58° C
B. 63.3° C
C. 68.7° C
D. 75.8° C
15. The total number of possible structural isomers of heptane is:
A. three.
B. seven.
C. nine.
D. twelve.