The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Alkenes
A. INTRODUCTION
Alkenes are hydrocarbon compounds that contain carbon-carbon double bonds. The first member of the alkene family is ethene (ethylene), similar to ethane of the alkane family in terms of the basic name, though the ending is -ene rather than -ane. The general molecular formula of alkenes is CnH2n.
B. NAMING OF ALKENES
Ethene (Ethylene) CH2=CH2
Propene (Propylene) CH2=CH-CH3
From the third member (butene) of the alkene family onward, there is a need to specify the location of the double bond to recognize the correct structure denoted by the name. When naming alkenes, the numbering should begin from the carbon chain end which gives the double bond position the lowest number.
Watch the numbering of carbons in the following examples.
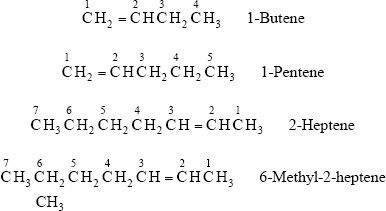
C. STRUCTURAL INTEGRITY AND ISOMERISM OF ALKENES
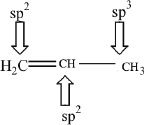
The carbon atoms which contain the double bonds in alkenes are sp2 hybridized. Each carbon-carbon double bond is made of one sigma and one pi bond.
Isomerism
Any compound with a carbon-carbon double bond can exhibit cis-trans isomerism, provided that the carbons involved in the double bond do not have two of the same groups or atoms attached to each of them. 2-Butene is the simplest alkene that can have cis-trans isomerism. Trans isomers are generally more stable than their cis counterparts. Alkyl groups are mildly electron-donating toward the double bond. This can lead to polarity. For instance, cis-2-butene has net dipole moment as shown in the diagram given below. On the other hand, in trans-2-butene the net dipole is zero since the dipole moments cancel out (the vector sum of the dipole moments is zero).
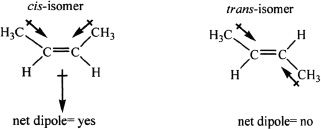
Both cis and trans-2-butene have van der Waals attractive forces. But, only the cis-isomer can have dipole-dipole interactions because it has a net dipole moment. Hencde, cis-2-butene has a higher boiling point than trans-2-butene.
The E-Z System of Naming Alkenes
Sometimes we are not able to categorize alkenes into trans and cis isomers. The following example will reveal why that is the case.
For compounds such as the ones shown above, we have to prioritize the substituents, and that is the only way to name and identify these compounds. A new system was proposed by scientists, which categorizes these compounds under E (entgegen), and Z (zusammen) configurations. E configuration describes opposite, whereas Z configuration describes same side. Study the following examples to get familiarized with this system of naming alkenes. In order to recognize which substituent is higher or lower, you have to compare their atomic numbers. That means an atom with a higher atomic number takes precedence over an atom with a lower atomic number.
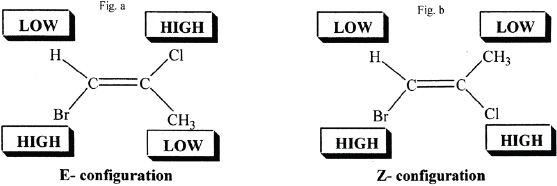
One key aspect to remember is that when we compare which substituent is higher or lower, we should compare the substitutions in the same carbon. In the above example, it is easy to see that on the left substitution, obviously bromine is higher (higher in the atomic number sense) than hydrogen. On the right side, chlorine is higher (atomic number:17) than carbon (atomic number:6). So the compound shown in Fig. (a) has an E configuration, and the one shown in Fig.(b) has a Z configuration.
D. GENERAL PROPERTIES OF ALKENES
Just like alkanes, the melting and boiling points of alkenes increase with an increase in the number of carbons (increase in chain length). Carbon-carbon double bonds are shorter than carbon-carbon single bonds. Alkenes are insoluble in water, but are soluble in nonpolar solvents such as hexane, and ethers
E. SYNTHESIS OF ALKENES
We can synthesize alkenes by processes such as dehydrogenation of alkanes, dehydrohalogenation of alkyl halides, and dehydration of alcohols.
From Alkanes
An alkene can be synthesized by the process of dehydrogenation (removal of hydrogen atoms), by heating an alkane up to about 700-7500.
Sample reaction 16-1
![]()
From Alcohols
When alcohols undergo dehydration reactions, alkenes are generated. There is a chance of rearrangement of the carbocation intermediates to form more stable carbocation intermediates, whenever possible, resulting in the formation of more than one type of alkenes. The mechanisms of such rearrangements are discussed in Chapter 21.
Sample reaction 16-2
![]()
The only product in the above reaction is ethylene, since there is no rearrangement and more importantly, there is no need for rearrangement. This is because the intermediate formed in this reaction is a primary carbocation, and rearrangement cannot produce a more stable carbocation.
From Alkyl Halides by Dehydrohalogenation
Alkenes can be synthesized by reacting the corresponding alkyl halides with a suitable strong base that can abstract a proton from one of the carbon atoms, while the leaving group attached to the adjacent carbon leaves. The general mechanism is shown below.
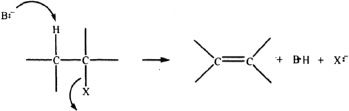
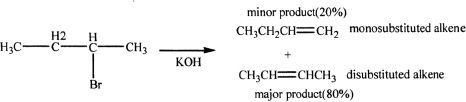
According to Zaitsev’s rule, the major alkene product is the one that is the most highly substituted. Consider the following example involving 2-bromobutane with potassium hydroxide.
Sample reaction 16-3
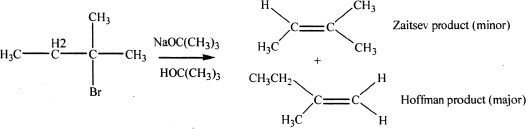
If bulky bases are used, the least substituted alkene may predominate as the product. In the next example, the base used is tert-butoxide ion. Since it is a bulky base it will preferentially abstract the less hindered hydrogen, leading to the formation of the least substituted alkene—the Hofmann product.
Sample reaction 16-4
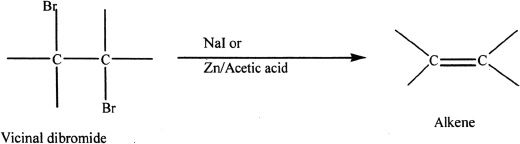
From Vicinal Dibromides by Dehalogenation
Vicinal dibromides have two bromines on adjacent carbon atoms. Vicinal dibromides can be converted to alkenes by reduction with zinc or iodide ion. A sample reaction is given below.
Sample reaction 16-5
F. REACTIONS OF ALKENES
Hydrogenation
In hydrogenation reactions, H2 is added to the unsaturated (carbon-carbon double or triple bonds) bonds. The resulting product of hydrogenation of a pure alkene is an alkane.
![]()
Usually, catalysts like platinum, palladium, or nickel are used for these types of hydrogenation reactions. Hydrogenation reactions are exothermic. Thus heat is generated as a result of hydrogenation and is called heat of hydrogenation.
The greater the substitution of the carbon-carbon double bond is, the lesser the heat of hydrogenation, and the higher the stability of the alkene.
Alkenes with Hydrogen Halides
Alkenes undergo electrophilic addition reactions with hydrogen halides, to form alkyl halides.
Sample reaction 16-6
![]()
In the process, the hydrogen halide attacks the double bond in the alkene, and the pi electrons in the double bond are transferred to the electrophile, resulting in a carbocation intermediate. This is followed by the formation of the alkyl halide.
![]()
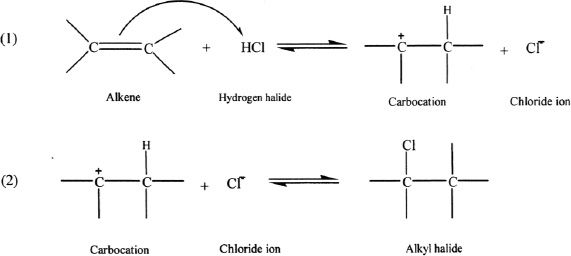
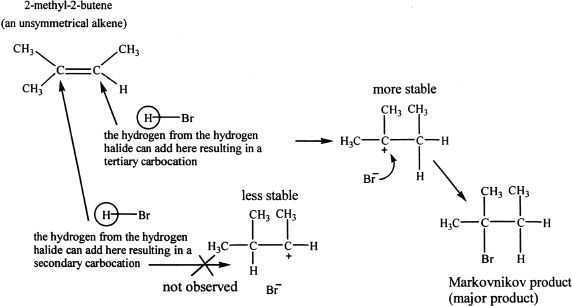
If the alkene used is not symmetrical, the possibility of different products from hydrogen halide addition is an issue. In such cases, the hydrogen from the hydrogen halide adds to the double-bonded carbon that already has the greater number of hydrogens. This is Markovnikov’s rule. Based on this rule, we can predict the major product in such reactions. Consider the following reaction involving 2-methyl-2-butene with hydrogen bromide.
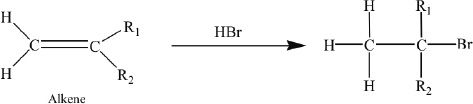
An example of a Markovnikov addition is shown below. Watch where the hydrogen and the bromine are added.
Sample reaction 16-7
Markovnikov addition of hydrogen and bromine
Sample reaction 16-8
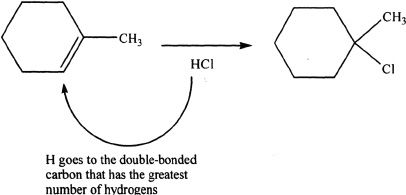
Anti-Markovnikov Addition
Hydrogen bromide in the presence of peroxides can add to an unsymmetrical alkene resulting in anti-Markovnikov products. The change in trend can be explained based on the mechanistic difference of HBr addition in the presence of peroxides. Peroxides can easily form free radicals, since the oxygen-oxygen bond in peroxides is weak. This type of addition is not seen with HCI or HI. The mechanism of HBr addition to an alkene in the presence of a peroxide is shown below.
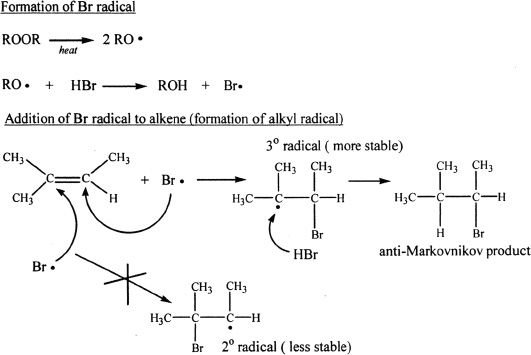
Notice that when the bromine radical adds to the double-bonded carbon that contains the most number of hydrogens, the resulting alkyl radical is more stable (here, tertiary radical is formed). Remember that free radical stability parallels carbocation stability. A tertiary radical is more stable than a secondary radical which is more stable than a primary radical.
Alkenes with Halogens
We will consider the reaction of ethylene with chlorine.
Sample reaction 16-9
The overall reaction:
![]()
The mechanism of the reaction:
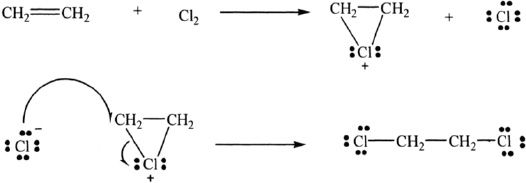
This is an example of electrophilic addition of Cl2 to an alkene. The mechanism of this reaction involves the following steps. In the first step, the ethylene reacts with chlorine to form the cyclic ethylene chloronium ion (intermediate) and chloride ion. Note that in this cyclic intermediate, the chlorine has a positive charge. This step is followed by the nucleophilic attack by chloride ion on the chloronium ion. The reaction is enhanced by electron-donating substituents such as alkyl groups on the carbon-carbon double bond, since such groups can further stabilize the formation of the transition state which results in the formation of the chloronium ion.
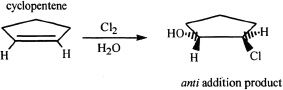
Halogen addition is usually an anti addition process. See the next reaction that exemplifies this aspect.
Sample reaction 16-10
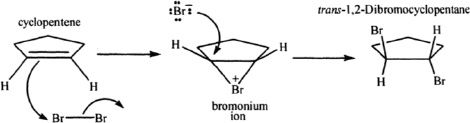
Alkenes with Halogens in Aqueous Medium
The organic product formed as a result of the reaction between an alkene and a halogen is called a halohydrin. An overall representative reaction is shown below:
Sample reaction 16-11
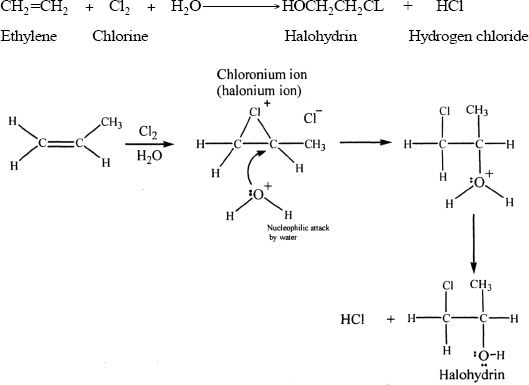
Sample reaction 16-12
Epoxidation
Sample reaction 16-13
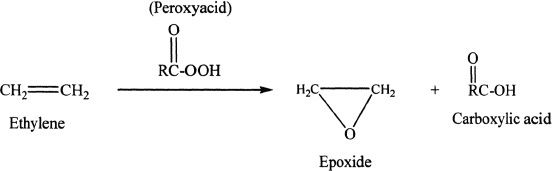
Alkenes react with peroxy acids to form epoxides and carboxylic acids as products.
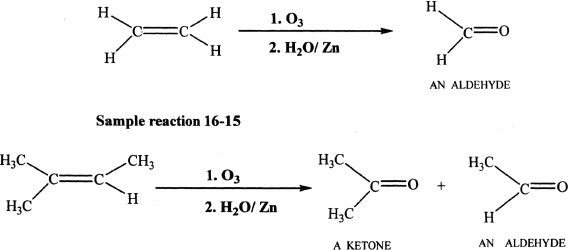
Ozonolysis
Alkenes react with O3 (Ozone) to form ozonides, which on hydrolysis with water form aldehydes or ketones or both, depending on the type of the reacting alkene. This is illustrated by the sample reactions.
Sample reaction 16-14
Hydroxylation using Osmium Tetroxide
Sample reaction 16-16
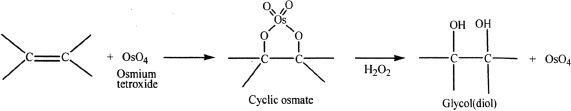
Osmium tetroxide can undergo reaction with alkenes to form a cyclic osmate, which in the presence of hydrogen peroxide results in a glycol (diol). Hydrogen peroxide oxidizes the osmium back to osmium tetroxide, while hydrolyzing the cyclic osmate to glycol. The predominant product is a syn addition product.
Acid Catalyzed Reaction
Sample reaction 16-17
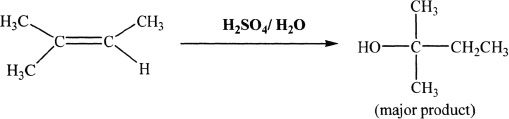
Alkenes react with aqueous acidic solutions to form alcohols. The reaction intermediate is a carbocation. There is possibility of rearrangement of the intermediates. The reaction follows Markovnikov’s rule.
Hydroboration-Oxidation
Sample reaction 16-18
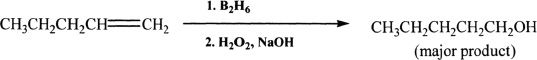
Oxidation followed by hydroboration of alkenes results in alcohols. This reaction takes place in an anti-Markovnikov fashion. Notice that the hydrogen atom, instead of attaching to the carbon contained in the double bond with the highest number of hydrogen substituents, attaches to the carbon with the least number of hydrogen substituents.
Diborane (B2H6), a dimer of borane (BH3), is usually used complexed together with tetrahydrofuran (THF) since diborane by itself is a toxic, and flammable gas. Borane entity actually adds to one of the double-bonded carbons resulting in an alkylborane. GH3 is a strong electrophile and adds to the least highly substituted double-bonded carbon. This preference makes sense because in the transition state, the electron deprived boron pulls electrons from the pi cloud resulting in a partial positive charge to the other carbon atom. This partial positive charge is better stabilized on the more highly substituted carbon. Hydrogen peroxide under basic conditions oxidizes the alkylborane to an alcohol. In effect, the addition in the hydroboration-oxidation is anti-Markovnikov.
Sample reaction 16-19
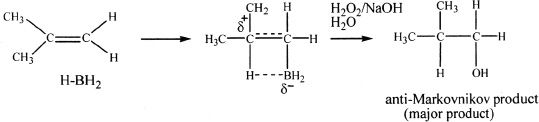
The addition of hydrogen and boron is simultaneous, and they must add to the same side of the double bond. Hence, this addition reaction involves syn addition or same-side addition. Study the next reaction.
Sample reaction 16-20
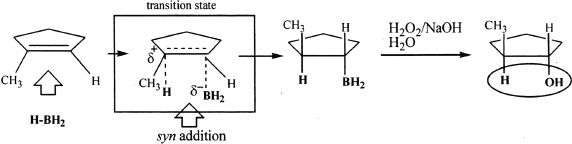
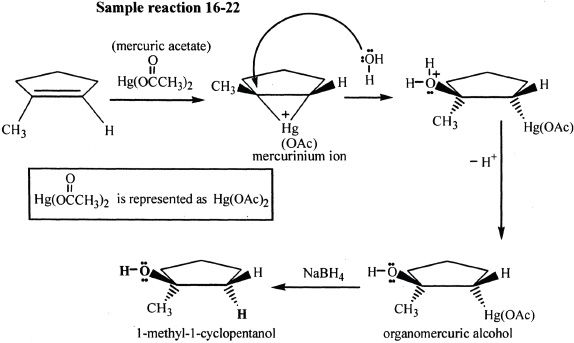
Oxymercuration-Demercuration
Sample reaction 16-21
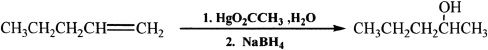
Alkenes can be converted into alcohols by oxymercuration-demercuration. The addition of H and OH is in accordance with the Markovnikov’s rule. There is no rearrangement of the intermediates in this process.
In the oxymercuation process, the electrophilic addition of the mercuric species occurs resulting in a mercurinium ion which is a three-membered ring. This is followed by the nucleophilic attack of water and, as the proton leaves, an organomercuric alcohol (addition product) is formed. The next step, demercuration, occurs when sodium borohydride (NaBH4) substitutes the mercuric acetate substituent with hydrogen. If an alkene is unsymmetric, Oxymercuration-demercuration results in Markovnikov addition. The addition of mercuric species and water follows an anti (opposite side) addition pattern. This reaction has good yield, since there is no possibility of rearrangement unlike acid-catalyzed hydration of alkenes.
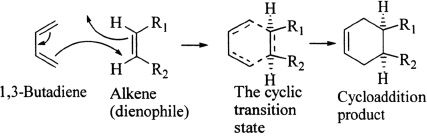
The Diels-Alder Reaction
The Diels-Alder Reaction is an addition reaction involving an alkene and a diene. Let’s look at the representative Diels-Alder reaction involving 1,3-butadiene and an alkene (dienophile means diene-lover). The reaction involves a cyclic transition state. The product is usually a cyclic addition product. Study the representative reaction given below. Pay close attention to how the new bonds are formed in relation to the reactants.
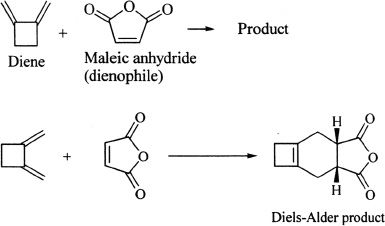
Notice that the substituents in the alkene remain the same way in the product. In other words, cis substituents remain cis in the cycloaddition product. Hence, the Diels-Alder reaction is stereospecific.
Sample reaction 16-23
CHAPTER 16 PRACTICE QUESTIONS
1. All the following are possible products of the acid-catalyzed dehydration reaction of the compound shown below, except:
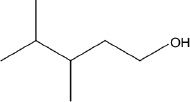
A. 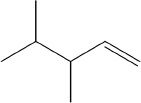
B. 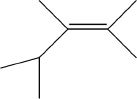
C. 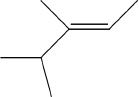
D. 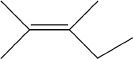
2. A student researcher calculated the number of moles of hydrogen used per mol for the hydrogenation of an unsaturated (only double bonds) aliphatic non-cyclic hydrocarbon compound. If the number of moles of hydrogen used for the complete hydrogenation of each mol of the hydrocarbon is eight, how many double bonds were there in the compound that was hydrogenated?
A. two
B. four
C. eight
D. sixteen
3. Choose the product of the following reaction.
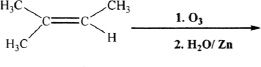
A. ![]()
B. 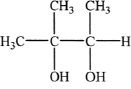
C. 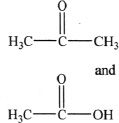
D. 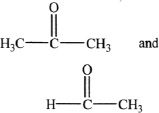
4. Which of the following is the most likely product of the reaction indicated below?
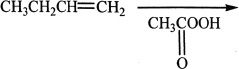
A. 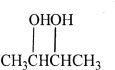
B. ![]()
C. 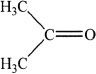
D. none of the above
5. Which of the following is the major product of the hydroboration-oxidation of 1-butene?
A. ![]()
B. ![]()
C. ![]()
D. ![]()
6. Which of the following compounds has the highest heat of hydrogenation?
A. ethylene
B. propene
C. 1-butene
D. 1-hexene
7. The major product that results in a reaction involving 1-propene with hydrogen bromide, in the presence of peroxides is:
A. 1-bromopropane
B. 1,2-dibromopropane
C. 2-bromopropane
D. 1,3-dibromopropane
8. Which of the following is true regarding a reaction of cis-2-pentene with KMnO4?
A. A trans diol will be formed.
B. A cis diol will be formed.
C. The reagent is not strong enough to oxidize cis-2-pentene.
D. The double bond will be broken, and is followed by a ring closure or ring formation.
9. In the acid catalyzed dehydration reaction of an alcohol, which of the following aspects listed can definitely be true?
A. There can be no rearrangement of the intermediate, regardless of the alcohol involved.
B. There can be rearrangement involved, resulting in less stable intermediates from more stable intermediates.
C. If possible, the major product is the most substituted product because of its higher stability.
D. None of the above.
10. In the acid-catalyzed dehydration of alcohols to alkenes, which of the following are true?
I) A carbanion intermediate is involved.
II) A carbocation intermediate is involved.
III) There is no intermediate involved.
IV) Methyl shifts can occur in the interme-diates.
V) Hydride shift can occur in the intermediates.
A. I, IV & V only
B. II, IV & V only
C. III only
D. II & V only
Questions 11-15 are based on the following passage.
Passage 1
The following synthesis reactions involving alkenes were done in a lab.
Reaction 1
![]()
Reaction 2
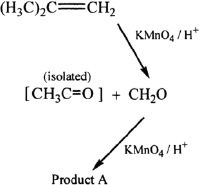
Reaction 3
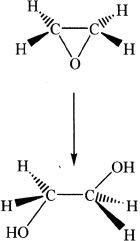
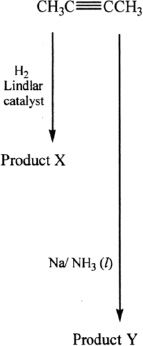
Reaction 4
11. Which of the following can be the oxidation product of an alkene?
A. CH3CH2CHO
B. HOCH2CH2OH
C. CH3CH2CH2COOH
D. All the above
12. Ethylene oxide in the presence of H2O can be converted to ethylene glycol by oxidation. In the process, the ring in ethylene oxide is attacked by water. Which of the following best describes the role of water?
A. Electrophile
B. Nucleophile
C. Merely a solvent
D. No active part in the reaction other than being a spectator molecule
13. In Reaction 2, what is the identity of Product A?
A. Acetic acid
B. Carbon dioxide
C. Methane
D. None of the above
14. Products X and Y in Reaction 4 are best described as:
A. mirror images.
B. tautomers.
C. anomers.
D. geometrical isomers.
15. What is the correct name of the compound shown below?
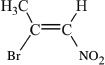
A. (E)-2-bromo-1-nitropropene
B. (Z)-2-bromo-1-nitropropene
C. (E)-2-bromo-1-nitrobutene
D. (Z)-2-bromo-1-nitrobutene