The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Alkynes
A. INTRODUCTION
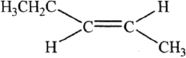
Alkynes are hydrocarbons that contain carbon-carbon triple bonds. The general formula of alkynes is CnH2n—2.
![]()
In acetylene, the carbon is sp hybridized. The triple bond is composed of one sigma bond, and two pi bonds. The bond angle is 180°. We should also realize that the sigma bonds are formed by sp hybrid orbitals, and the pi bonds are formed by the p orbitals.
If the triple bond in an alkyne is at the end of a carbon chain, it is called a terminal alkyne. If the triple bond is not at the end of a carbon chain, it is called an internal alkyne.
![]()
B. NAMING OF ALKYNES
The naming of alkynes is similar to that of alkenes.
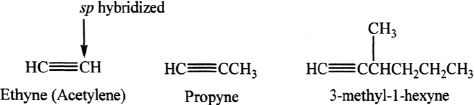
C. SYNTHESIS OF ALKYNES
Acetylene Synthesis by Adding H2O to CaC2 (calcium carbide)
Sample reaction 17-1
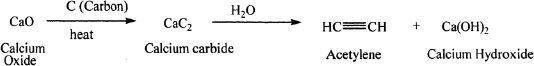
From Dihalogenoalkanes
Sample reaction 17-2
A representative reaction is given below:
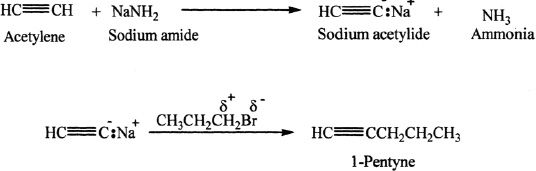
From Acetylene
Higher alkynes can be synthesized from acetylene by reacting with NaNH2 followed by treatment with the appropriate alkyl halide. Terminal alkynes can be deprotonated using strong bases such as sodium amide. Hydroxides or alkoxides are not strong enough to deprotonate an acetylenic hydrogen.
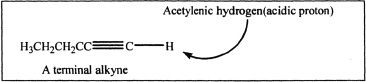
Sample reaction 17-3
D. REACTIONS OF ALKYNES
Hydrogenation
In the presence of catalysts such as palladium, platinum, or nickel, alkynes can be hydrogenated to the corresponding alkanes.
Sample reaction 17-4
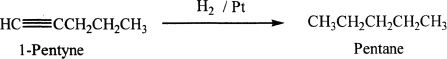
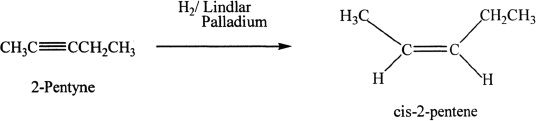
Alkynes can be hydrogenated to alkenes by using specific catalysts such as Lindlar palladium. This reaction is stereoselective resulting in syn addition.
Sample reaction 17-5
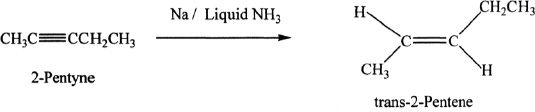
An alkyne can be hydrogenated to a trans-alkene by hydrogenating the alkyne with sodium in liquid ammonia.
Sample reaction 17-6
Acid-Catalyzed Hydration
Alkynes can undergo acid-catalyzed hydration reactions. The product is an enol, which is an unstable intermediate, and is immediately converted to a ketone. The formation of enol is based on Markovnikov addition (hydration).
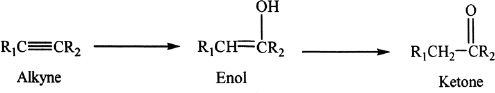
Sample reaction 17-7
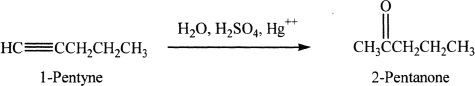
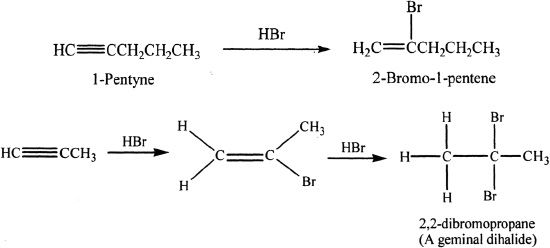
Alkynes with Hydrogen Halides
Alkynes can undergo reactions with hydrogen halides. The reaction follows Markovnikov’s addition of hydrogen and halogen to the triple bond. When two moles of hydrogen halide gets added to an alkyne, the reaction follows the same Markovnikov’s pattern resulting in a geminal dihalide (two halogen atoms attached to the same carbon atom). Study the reactions given below.
Sample reaction 17-8
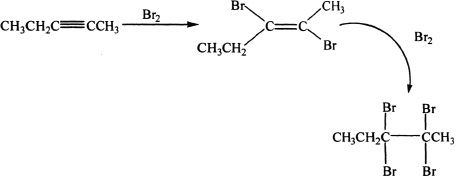
Alkynes with Halogens
Alkynes can undergo reactions with halogens forming di- and tetra-halogenated products.
Sample reaction 17-9
Ozonolysis
Alkynes can undergo ozonolysis. The products formed are carboxylic acids.
Sample reaction 17-10
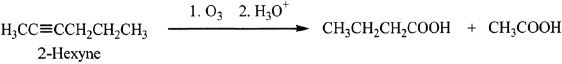
In this reaction, the products formed are butanoic acid and acetic acid. If a terminal alkyne is used, the products are carbon dioxide and a carboxylic acid. See the example given below.
![]()
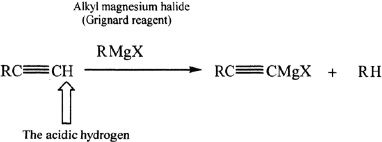
Reaction Involving the Acidic Hydrogen in an Alkyne
The acidic hydrogen present in terminal alkynes can undergo reactions with Grignard reagents. A sample reaction is shown.
Sample reaction 17-11
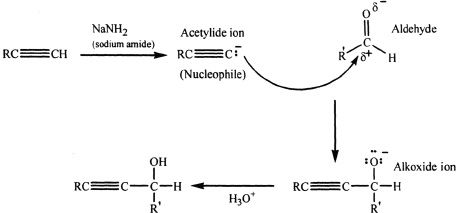
Acetylide with Aldehydes and Ketones
Acetylides are strong bases and are good nucleophiles. So they can add to the carbonyl groups in aldehydes and ketones. The alkoxide ion that is formed during the reaction can be protonated to an alcohol by treating it with aqueous dilute acid.
CHAPTER 17 PRACTICE QUESTIONS
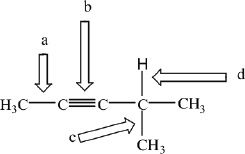
1. In the compound shown above, which of the indicated bonds (arrows) have the shortest carbon-carbon bond?
A. a
B. b
C. c
D. d
2. If bottle A contains cis-2-butene, bottle B contains 1-propyne, and bottle C contains butane, identify the bottle that contains the compound with carbon-carbon bonds of the highest bond energy.
A. Bottle A
B. Bottle B
C. Bottle C
D. They all have equal bond dissociation energies.
3. Which of following compounds has the lowest pKa value?
A. Ethane
B. Ethene
C. Acetylene
D. None of the above listed compounds are strong acids, and hence they do not have pKa values
![]()
4. The compound shown above is treated with hydrogen in the presence of Lindlar catalyst. The major product is:
A. pentanol.
B. cis-2-pentene.
C. pentane.
D. trans-2-pentene.
5. 1-Butyne is treated with aqueous sulfuric acid in the presence of mercuric oxide as the catalyst. The major product is:
A. 1-Butanone.
B. 2-Butene.
C. 2-Butanone.
D. 1-butanol.
6. Which of the following choices represents the product of ozonolysis of 3-Hexyne?
A. Propanone
B. Propanal
C. Hexanoic acid
D. Propanoic acid
7. Double dehydrohalogenation of an alkyl vicinal dihalide can directly result in:
A. a carboxylic acid.
B. an alkyne.
C. an alcohol.
D. an ester.
8. The process by which an alkyne can be directly converted into an alkane is called:
A. dehydrohalogenation.
B. dehydration.
C. hydrolysis.
D. hydrogenation.
9. If liquid ammonia in the presence of sodium is added to an alkyne, the major product is:
A. a trans-alkene.
B. a cis-alkene.
C. an aldehyde.
D. a carboxylic acid.
10. Which of the following is true regarding alkynes?
A. Alkynes have generally higher boiling points than corresponding alkanes.
B. Alkynes have sp hybridized carbons.
C. Acetylene (an alkyne) can form salts called acetylides when exposed to strong bases.
D. All the above are true.
Questions 11-16 are based on the following passage.
Passage 1
Alkynes are hydrocarbons with carbon-carbon triple bonds in them. The simplest alkyne can be prepared by the process shown in Reaction 1.
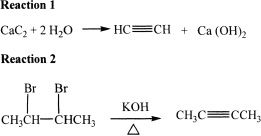
Also consider the following reactions.
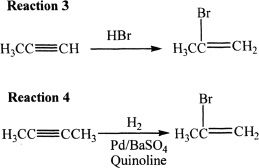
Quinoline is a heterocyclic amine.
11. The hybrid orbital that characterizes and differentiates alkynes from other simple hydrocarbons like alkanes and alkenes, is that alkynes have hybrid orbitals which have:
A. 75% p character and 25% s character.
B. 66% p character and 33% s character.
C. 50% p character and 50% s character.
D. none of the above.
12. Reaction 3 is best described as:
A. a substitution reaction.
B. an addition reaction.
C. an elimination reaction.
D. a dehydrogenation reaction.
13. Which of the following sequences best describes a way to synthesize 1-hexyne from acetylene?
A. 1. NaNH2/liq. ammonia
2. n-propyl bromide
B. 1. NaNH2/liq. ammonia
2. tert-butyl bromide
C. 1. n-Propyl bromide
2. NaNH2/liq. ammonia
D. 1. NaNH2/liq. ammonia
2. n-butyl bromide
14. Lindlar’s catalyst is often used for the reduction of alkynes to alkenes. The catalyst consists of palladium which is deposited in a finely divided state of barium sulfate, which is subsequently treated with a heterocyclic amine called quinoline. Which of the following is the most likely function of quinoline?
A. To speed up the reaction so that the alkyne is completely converted to its most reduced form of hydrocarbon
B. To slow down the reaction, because the function of a catalyst is to slow down the reaction
C. To moderate the catalytic activity to facilitate a restrictive reduction
D. It acts as an oxidizing agent so that the alkyne is reduced.
15. Which of the following is the most likely structure of quinoline?
A. 
B. 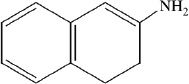
C. 
D. 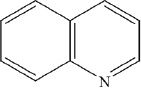
16. Predict the major product of the reaction shown below:
![]()
A. ![]()
B. 
C. 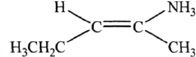
D.