The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Aromatic Compounds
A. INTRODUCTION
Aromatic compounds are compounds which have benzene rings in them. Aromatic hydrocarbons are also called arenes. Some compounds have structures which look like fused benzene rings. Such compounds are called polycyclic aromatic compounds.
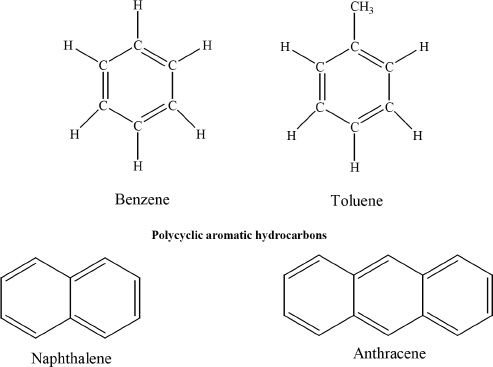
B. BENZENE
Structure of Benzene
The formula of benzene is C6H6. The ring structure has alternate double and single bonds as shown in the diagrams below. According to the latest theories, benzene is in resonance between the two structural entities as shown below. Benzene has a structure that is a hybridized form of these two structures.
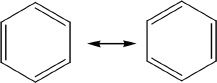
The hybridized structure is usually represented as shown below:
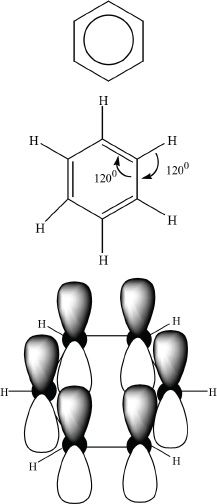
Figure 18-1
The six carbons in the benzene ring have a planar arrangement of a regular hexagon. The carbons are located at the vertices of the hexagon. The carbon-carbon bonds have the same length, and each bond angle is 120°. The hybridization is sp2. The sigma bonds are formed by the sp2 hybridized orbitals. However, the unhybridized 2p orbitals in each carbon overlap to form a cyclic pi system around the ring. The delocalization of these electrons creates the pi system in aromatic compounds. The electron cloud of the pi system occupies the regions above and below the ring as depicted in Figure 18-1.
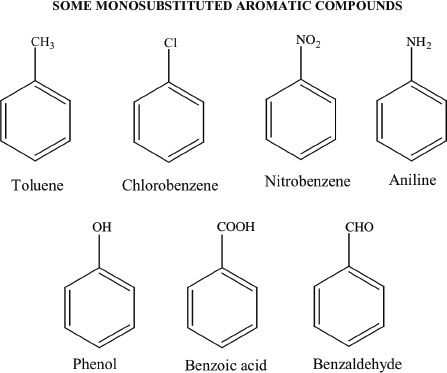
C. DERIVATIVES OF BENZENE
Many derivatives of benzene are substituted derivative compounds. Some examples of such compounds are shown below:
D. PROPERTIES OF AROMATIC COMPOUNDS
Aromatic hydrocarbons are nonpolar, and are insoluble in water. They commonly undergo reactions like aromatic electrophilic substitution reactions and reduction reactions.
E. CONCEPT OF AROMATICITY
Aromaticity of compounds is based on certain rules. According to Huckel’s rule, the number of pi electrons present should be a number denoted by the formula 4n + 2, where n is 0, 1, 2, 3, …. Hence, for compounds to be considered aromatic the number of pi electrons should be 2, 6, 10, …. In addition, the structure should also have a cyclic pi system to be aromatic. Atoms in the ring must have unhybridized p orbitals. These unhybridized p orbitals must overlap resulting in a cyclic pi system. For this overlap, the structure must have planar (or nearly planar) configuration. In other words, the atoms related or involved in the pi bond should be in the same plane.
Test whether you can recognize the aromaticity of the structures in the examples that follow:
Tips for testing aromaticity
Step 1 — Count the number of pi electrons.
Step 2 — See whether there is a cyclic p orbital overlap.
Predict the aromaticity of the compounds given below:
Compound 1
Number of pi electrons — 6
Cyclic overlap of p orbitals — Yes
Aromatic — Yes
Compound 2
Number of pi electrons — 4
Cyclic overlap of p orbitals — No
Aromatic — No
Compound 3
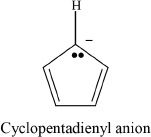
Number of pi electrons — 6
Cyclic overlap of p orbitals — Yes
Aromatic — Yes
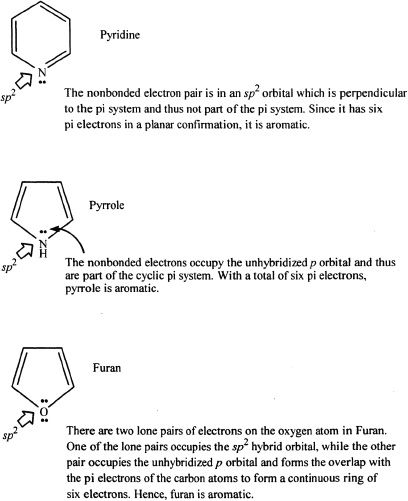
Some examples of heterocyclic aromatic compounds.
F. REACTIONS OF AROMATIC COMPOUNDS
Benzene Stability
Benzene, unlike alkenes, will not react with halogens to form addition products.
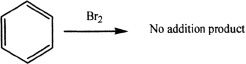
This exemplifies the extra stability of the double bonds present in the benzene ring. However, benzene can undergo substitution reactions with halogens in the presence of a Lewis acid catalyst. The Lewis acid enhances the electrophilic nature of the halogen, thus enabling the reaction to proceed.
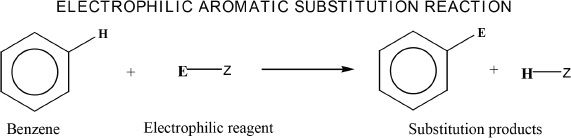
Electrophilic Substitution Reactions
One of the most characteristic reactions of benzene is the electrophilic substitution reaction, in which a hydrogen is replaced by an electrophile. In such reactions, the benzene acts as the nucleophile.
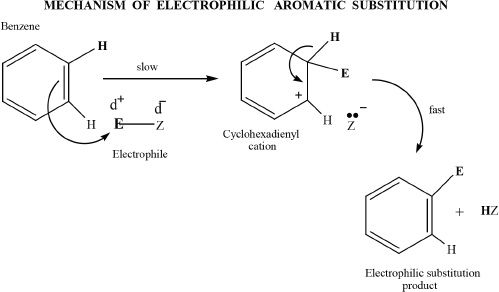
In a typical aromatic substitution reaction, the electrophile (means electron loving) accepts the electron pair from the pi system of benzene, resulting in a carbocation. This cation of benzene is called the cyclohexadienyl cation. Then, the cyclohexadienyl cation loses a proton forming the substitution product.
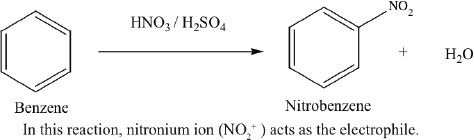
Nitration
Benzene can be nitrated by reacting with nitric acid (HNO3). This is usually done in the presence of sulfuric acid (H2SO4).
Sample reaction 18-1
Friedel-Crafts Reactions
In Friedel-Crafts reactions, benzene is reacted with acyl or alkyl chlorides, in the presence of metal halides as catalysts. The metal halides act as Lewis acids in these reactions. The two types of Friedel-Crafts reactions are alkylation and acylation.
(1) Alkylation
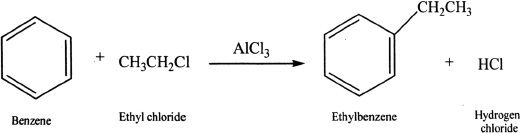
In Friedel-Crafts alkylation, benzene is reacted with alkyl chlorides in the presence of metal halides (AlCl3, AlBr3) as catalysts. The catalyst serves as Lewis acid and increases the electrophilicity of the alkyl halide. Alkylation is important for the synthesis of alkyl substituted derivatives of benzene. But there is a possibility of rearrangement of the intermediates resulting in undesired products. For example, if primary halides are used in alkylation, they can rearrange to form secondary or tertiary carbocations which are more stable intermediates. This can result in multiple products.
Sample reaction 18-2
The Mechanism of Alkylation Rearrangement
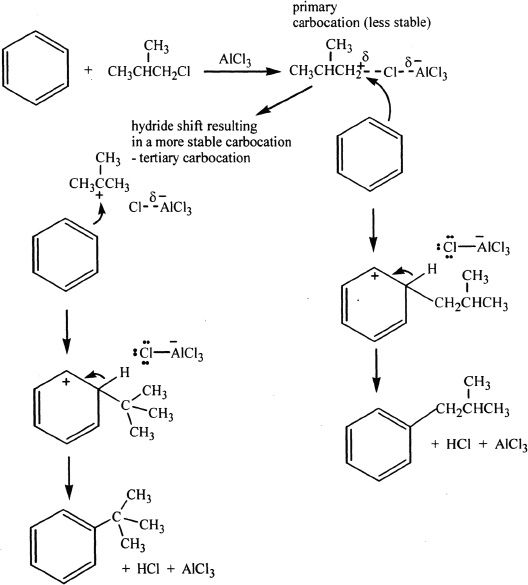
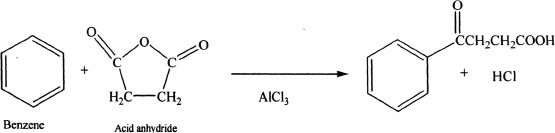
(2) Acylation
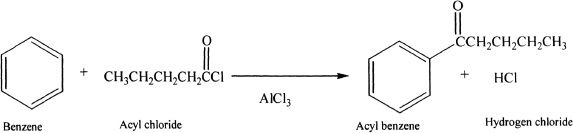
In Friedel-Crafts acylation reactions, benzene is reacted with acyl chlorides or acid anhydrides in the presence of metal halides (Lewis acids). The importance of acylation is that there is no rearrangement, unlike alkylation where there is a possibility of rearrangement of the cation intermediates.
The acyl halide forms a complex with the Lewis acid (AICI3), followed by the leaving of the halogen along with the Lewis acid. The resulting ion, called acylium ion, is resonance stabilized and is strongly electrophilic. The ion reacts with benzene to form an acylbenzene.
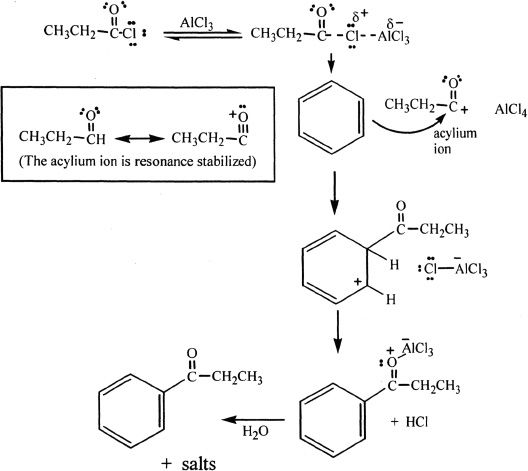
Sample reaction 18-3
Acyl products that are formed can be reduced by reactions such as Clemmenson or Wolf-Kishner reductions.
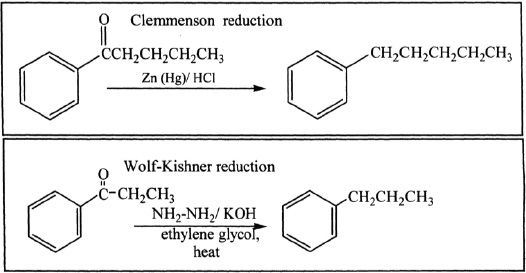
Sample reaction 18-4
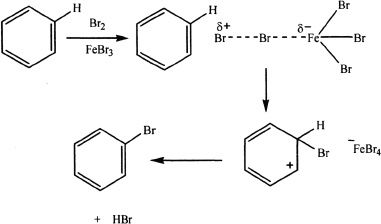
Halogenation
Sample reaction 18-5
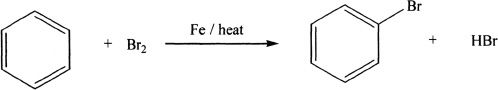
Benzene can be halogenated in the presence of Fe. In this reaction, the iron(III) bromide acts as the catalyst, which is formed from the iron and the bromine. The bromine-iron(III) bromide complex that is formed acts as the electrophile which attacks the benzene.
Sulfonation
Benzene can be sulfonated by heating with sulfuric acid.
Sample reaction 18-6
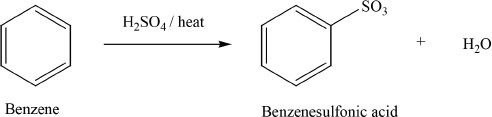
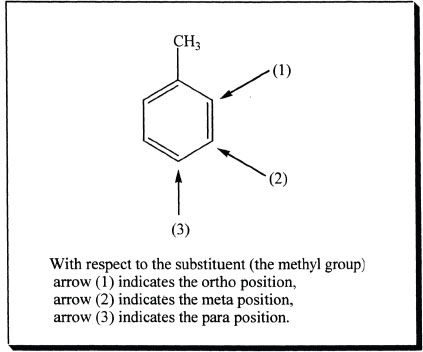
G. DIRECTIVE EFFECTS OF SUBSTITUENTS
Consider the reaction of benzene with an electrophile (E).
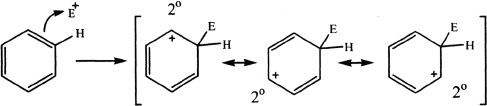
Notice that the positive charge is spread only on secondary carbons, and thus all resonance structures are equally stable.
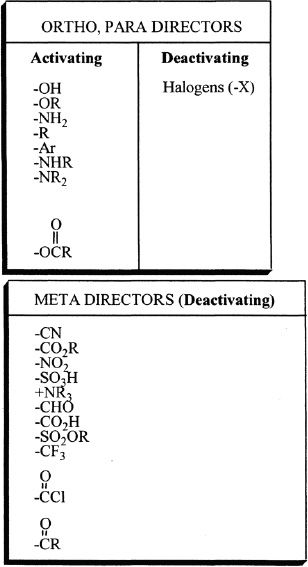
In aromatic substitution reactions, the groups already present in the benzene ring can significantly influence the place of electrophilic attack of the incoming substituent. In other words, the substitution is influenced by the groups that are already present in the benzene ring. There are two types of groups — activators and deactivators. The groups that are activators cause the ring to be increasingly reactive than benzene. The groups that are deactivators cause the ring to be decreasingly reactive than benzene.
Activating Groups
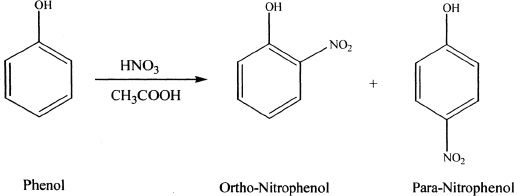
As mentioned above, the activators increase the reactivity of the ring than that of benzene. In addition, the substitution primarily occurs in the ortho and para positions of the ring relative to the activating group. For this reason, activators are also called ortho/para directors. Let us analyze this with an example. In the following reaction, phenol is nitrated. Notice that phenol contains a hydroxyl group (-OH) which is an ortho/para activator. So the substitution occurs at ortho and para positions with respect to the -OH group.
Sample reaction 18-7
Now let’s us consider an electrophilic attack on toluene.
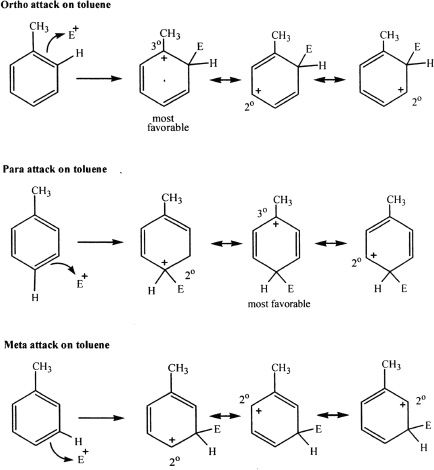
Because the attack on the ortho or para attack results in more stable cation intermediates, those intermediates are formed faster and thus the resulting products from those intermediates are the predominant products.
By observing the resonance forms of the cation intermediates, it is clear that ortho and para attacks are favored. This is because ortho and para attacks have the possibility of spreading or sharing the positive charge by a tertiary carbon. Notice that when the attack is at the meta position, the positive charge is only shared by secondary carbons. A positive charge is better stabilized when it is on a tertiary carbon than on a secondary carbon. The methyl substituent is electron-donating and activates the benzene ring toward electrophilic attack, and the activation is more towards the ortho and para positions than the meta positions. In other words, alkyl groups are electron-donating substituents that are ortho-para directing by donating the electron density and thereby inductively stabilizes the intermediate.
Alkyl groups are electron-donating substituents, but it might seem counterintuitive to think at groups like -OH (hydroxyl) and -OCH3 (methoxy) are also ortho/para activators since oxygen is highly electronegative atom. This is because the oxygen atom that is bonded to the ring carbon has lone electrons or nonbonding electrons. As a result, the positive charge on the carbon atom is the intermediates formed from ortho and para attacks is stabilized through resonance. In other words, such groups can also donate electron density because of the presence of nonbonded electrons.
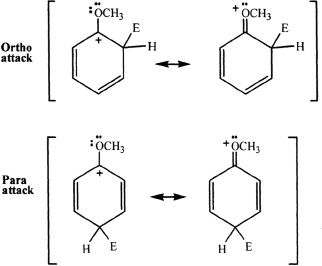
Deactivating Groups
Deactivators make the ring less reactive than benzene. There are two types of deactivators — ortho/para and meta deactivators.
1) Ortho/para deactivators
Some deactivators direct the incoming substituents primarily to the ortho and para positions. Halogens (Fluorine, Chlorine, Bromine, Iodine) are ortho/para deactivators. They are electron withdrawing substituents through inductive effect. Furthermore, they have nonbonded electrons that can donate electron density by resonance. Because of the high electronegativity of halogens, they pull the electron density away from the ring, making the ring less susceptible to electrophilic substitution (deactivating property). Halogen substituents have nonbonded electrons which can be donated to a positively charged carbon in the intermediates resulting from ortho and para attacks. Hence, halogens are ortho, para-directing, and deactivating substituents.
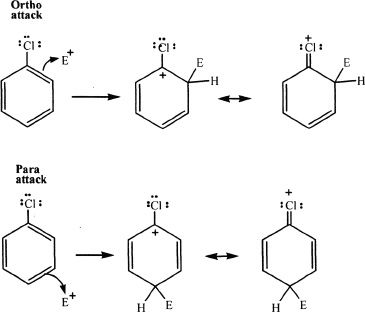
2) Meta deactivators
The meta deactivating groups direct the incoming substituents to the meta position. These groups are strongly electron-withdrawing groups. Consider the following example.
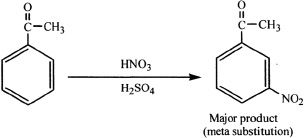
The substituent acyl group has a highly polarized carbon-oxygen double bond.
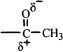
The positively charged carbon withdraws electron density from the benzene ring inductively. This accounts for the deactivation (less reactivity than benzene) of the ring toward electrophilic substitution.
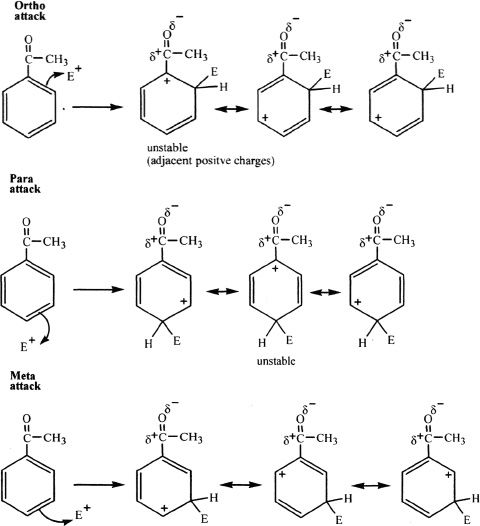
Both ortho and para attacks result in an intermediate that has adjacent positively charged (polarized) atoms, making it a highly unstable (higher energy) form. On the other hand, meta substitution doesn’t result in an intermediate with positively charged atoms adjacent to each other. Thus, meta substitution results in the most stable (lower energy) intermediates. This accounts for the predominance of meta substitution products if acyl or similar groups are present in the ring.
Table 18-1
CHAPTER 18 PRACTICE QUESTIONS
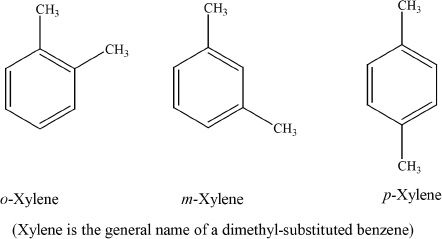
1. Which of the following compounds is commonly known as xylene?
A. 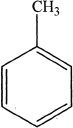
B. 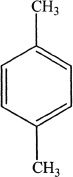
C. 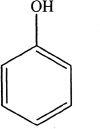
D. 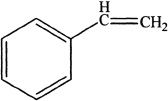
2. The carbons in the benzene ring are:
A. sp hybridized.
B. sp2 hybridized.
C. sp3 hybridized.
D. not hybridized.
3. All the following are monosubstituted aromatic compounds, except:
A. phenol.
B. benzoic acid.
C. xylene.
D. toluene.
4. A compound can be aromatic, only if it has a certain number of pi electrons in its ring. All the following numbers can be attributed to the number of pi electrons and aromaticity, except:
A. two.
B. four.
C. six.
D. ten.
5. For the reaction involving benzene and nitric acid in the presence of sulfuric acid, which of the following is true?
A. The nitronium ion acts as the electrophile.
B. The reaction is an example of nucleophilic hydrogenation.
C. The reaction is an example of electrophilic addition reaction.
D. None of the above
6. What is the name of the reaction shown below?
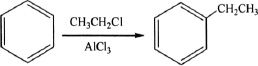
A. Clemmenson reaction
B. Williamson synthesis
C. Raman synthesis
D. Friedel-Crafts reaction
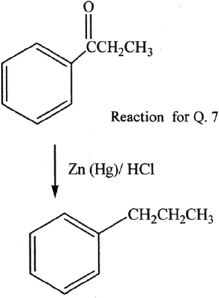
7. The reaction shown is commonly known as:
A. Clemmenson reduction.
B. Wolff-Kishner reaction.
C. Benedicts reaction.
D. Zaitsev reaction.
8. The group -NO2 can be best described as:
A. an ortho/para activator.
B. a meta activator.
C. an ortho/para deactivator.
D. a meta deactivator.
9. Halogens can be best described as:
A. ortho/para activators.
B. meta activators.
C. ortho/para deactivators.
D. meta deactivators.
10. If the compound shown below was reacted with nitric acid in the presence of sulfuric acid, which of the following is the major product?
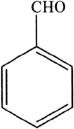
A. A para substituted product
B. A meta substituted product
C. An ortho substituted product
D. No net reaction occurs
Questions 11-16 are based on the following passage.
Passage 1
The earliest predictions about the structure of benzene could not completely explain some of its properties. Later it was found that benzene has a cyclic structure which is commonly represented as follows:
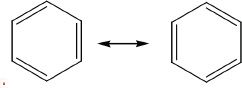
These two structures are resonance structures of benzene. Benzene is also commonly represented as shown below:
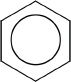
A series of reactions were conducted using benzene and other aromatic compounds.
Reaction 1
In Reaction 1, benzene was reacted with n-propyl chloride in the presence of AlCl3.
Reaction 2
Nitrobenzene was reacted with nitric acid and sulfuric acid. The reaction occurred as follows:
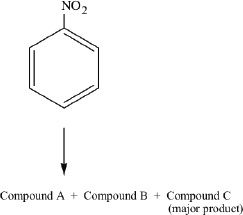
11. Which of the following is true regarding Reaction 1 described in the passage?
A. It is a Friedel-Crafts acylation reaction.
B. It is a reaction which results in a single product.
C. AlCl3 acts as a Lewis base.
D. Rearrangement is possible.
12. Alkoxy group is best described as:
A. an ortho, para-director, and an activating group.
B. an ortho, para-director, and a deactivating group.
C. a meta-director, and an activating group.
D. a meta-director, and a deactivating group.
13. If phenol is reacted with bromine, the major product formed is:
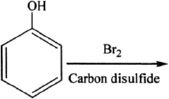
A. 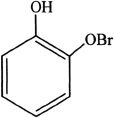
B. 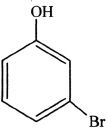
C. 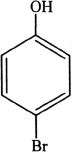
D. 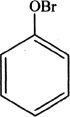
14. The reaction shown here is:
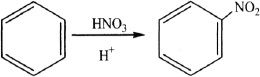
A. an electrophilic substitution reaction.
B. an electrophilic addition reaction.
C. a reduction-addition reaction.
D. an electrophilic elimination reaction.
15. The inscribed circle in the benzene ring represents:
A. the carbon chain in the cyclic ring.
B. the sigma-system of electrons.
C. the pi-system of electrons.
D. the eight pi-electrons in the cyclic system.
16. All the following are false about Reaction 2, except that:
A. Compound A is a meta product.
B. the major product is a 1,2 substituted product.
C. the major product is a para substituted product.
D. Compound C is a 1,3 substituted product.