The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Stereochemistry
A. INTRODUCTION
Stereochemistry is the study of the three-dimensional structures of compounds. In this chapter, we will discuss the various concepts in stereochemistry starting with some simple terms such as isomers. What are isomers? Isomers are compounds with the same molecular formula, but with different arrangements of atoms. Stereochemistry takes us further into the intricacies of isomerism in terms of three-dimensional perspectives. Furthermore, stereochemistry helps us understand the differences in activities of molecules that are structurally rather close. In many compounds, the slightest changes in the three-dimensional form of molecules make them active or inactive. This concept is especially important in many biologically significant molecules. Stereochemistry has taken us deep into the secrets of many naturally occurring molecules in our body.
Constitutional isomers have the same molecular formula but different arrangements. Look at the examples shown in Figure 19-1. They are constitutional isomers.
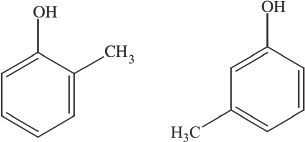
Figure 19-1
B. STEREOISOMERS
Isomers with atoms having the same order of bonding, but different spatial arrangements are called stereoisomers. Stereoisomers which are non-superimposable mirror images are called enantiomers. Diastereomers are stereoisomers that are not mirror images.
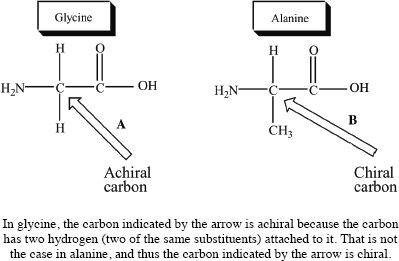
Figure 19-2
A carbon which is sp3 hybridized (tetrahedral structure) with four different substituents is called a chiral carbon. If the carbon doesn’t have four different substituents or say at least the carbon has two of the same substituents, then it is an achiral carbon.
Enantiomers
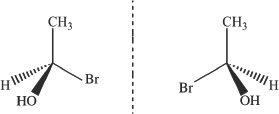
Enantiomers are compounds with same molecular formula and are nonsuperimposable mirror images. Look at the example shown in Figure 19-3.
Diastereomers
Diastereomers have the same molecular formula, but they do not have a mirror-image relationship to each other. Study the examples in Figure 19-4.
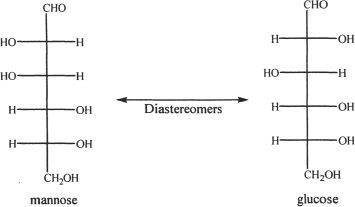
Figure 19-4
C. OPTICAL ACTIVITY
The molecular structure and the geometry of a compound dictate many of its properties. The structural integrity of certain molecules makes them capable of rotating the plane of polarized light. In order to make use of this phenomenon, we must have a source of plane polarized light. When light waves are passed through polarizing materials, the electric field vector of the processed light oscillates in one plane. This is plane polarized light.
To examine the chiral properties, experimenters pass plane polarized light through solutions of chiral compounds. The rotation of light is noted with a detector. If the rotation perceived by an observer looking through the solution toward the source of light is clockwise, positive (+) sign is used to denote the optical activity. For counterclockwise rotation, negative (—) sign is used to denote the optical activity. The positive rotation is often referred as d (dextrorotatory) and negative rotation as l (levorotatory).
Some Generalizations Regarding Optical Activity
Consider two solutions — A and B. Solution A contains a pure enantiomer, and solution B contains the enantiomer of the compound in solution A. Let’s say that solution A exhibited positive rotation.
Since the solution A containing a pure enantiomer exhibits positive rotatory properties, its enantiomer will have negative rotatory properties. What can we conclude about these compounds? Well, we can be certain that both compounds present in the solutions A and B are chiral, since they exhibit optical activity.
The specific rotation [α] of a compound at a given wavelength is denoted by
![]()
where αis the observed rotation,
c is the concentration in g/ml,
and 1 is the length (in decimeters) of the polarimeter tube (the optically active solution is taken in the polarimeter tube for the analysis) that is used.
Properties of Enantiomers and Diastereomers
Enantiomers have identical physical properties. So they cannot be distinguished based on their melting points, boiling points, and densities. But enantiomers do differ in terms of optical activity. Enantiomers rotate place polarized light with the same magnitude, but in opposite directions. Because enantiomers have identical physical properties, separation of enantiomers using conventional methods such as simple distillation and recrystallization is not possible. Enantiomers react the same way with achiral molecules, but react differently with chiral molecules. This difference in reactivity of enantiomers toward chiral molecules is utilized in separating enantiomers. The process is called resolution. The enantiomeric mixture is reacted with a chiral molecule to form a pair of diastereomers. Because diastereomers have different physical properties, they can easily be separated. Diastereomers usually have different solubilities. Followed by the separation of the diastereomers that are formed, the original reaction is reversed to get the original enantiomer corresponding to the diastereomer.
D. CONFIGURATIONS
Before we look at configurations, we need to study chirality and associated ideas.
Chirality and Achirality
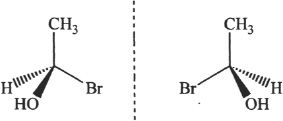
Figure 19-5
If a molecule does not have a plane of symmetry, it is chiral. A chiral molecule is not superimposable on its mirror image. The structures represented in Figure 19-5 are mirror images. These mirror images are non-superimposable. The molecules depicted in Figure 19-5 are chiral.
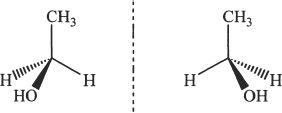
Figure 19-6
We will now consider Figure 19-6. The mirror images of the molecule depicted are superimposable. Hence it is an achiral molecule. Notice that two of the atoms attached to the central carbon atom are the same, namely the hydrogen atoms.
Achirality can also be recognized by looking at the molecular structure. When we are analyzing the structure of a molecule, we should look and determine whether there is any plane of symmetry in the molecule. Plane of symmetry reflects achirality.
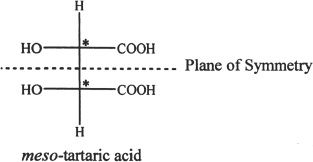
The figure given below shows a stereoisomer of tartaric acid. Notice that this compound has two chiral (stereogenic) centers. But, there is a plane of symmetry and thus the molecule itself is achiral and optically inactive. Such compounds that contain one or more stereogenic centers, but are achiral, are called meso compounds. Hence, having a stereogenic center or chiral carbon does not always lead to chirality of the entire molecule.
Absolute Configuration
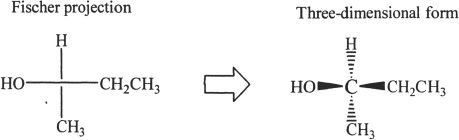
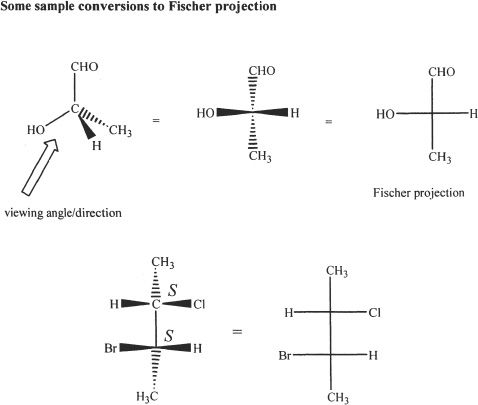
In Fischer projection, the horizontal lines represent bonds that are toward you, and the vertical lines represent bonds that are pointing away from you.
Absolute configuration is the arrangement of substituents around the stereogenic center of a chiral molecule. The Fischer projection and the absolute configuration of the amino acid alanine is shown in Figure 19-7.
R-S System of Representation
The R-S system of representation is a convenient and essential way of looking at molecules. The R-S convention is done by prioritizing the substituents that are bonded to the chiral carbon.
The following rules will familiarize you with the R-S naming of compounds.
1. The orientation of the molecule should be in such a way that the lowest priority group is pointing away from you.
2. First, prioritize each group that is bonded to the chiral carbon. The priority is based on atomic number. Higher the atomic number of the atom (in the group) that is connected to the chiral carbon, higher the priority of that group. For example, if the four groups connected to the chiral carbon are CH3,H, OH, and Br, then the bromine atom (atomic number 35) has the highest priority. This is followed by the oxygen atom (atomic number 8) of the hydroxyl group, then the carbon atom of the methyl group, followed by the hydrogen atom (atomic number 1). It is important to realize that the priority is determined by the atomic number of the atom that is directly connected to the chiral atom.
3. If two groups that are attached to a chiral carbon are isotopes (same atomic number, different mass numbers), the heavier isotope takes precedence.
4. If two groups have the same atom connected to a chiral carbon, then the next atom along the chain determines the priority. If that too fails (if it is the same atom), then go to the next atom to determine which group has higher priority. For example, -CH2F has a lower priority than -CH2I. If the groups contain unsaturations such as double or triple bonds, consider that the atoms on both ends are duplicated depending on the number of bonds.
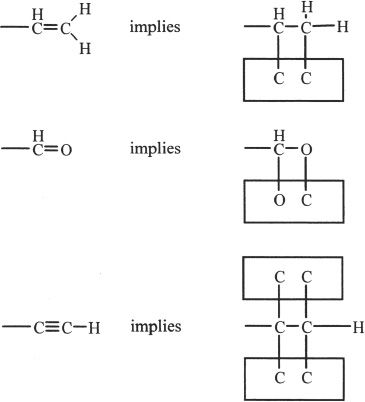
5. After prioritizing, draw an arrow starting from the first priority group to the third priority group through the second priority group. This is illustrated in the example given below.
6. If the arrow points in the clockwise direction, the configuration of that chiral carbon is R. If the arrow points in the counterclockwise direction, the configuration of that chiral carbon is S.
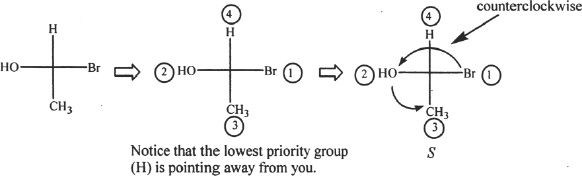
If the given orientation of a molecule shows the lowest priority group pointing toward the viewer, the orientation should be changed so that the lowest priority group points away form the viewer. In Fischer projection, if the lowest priority group is attached to a vertical bond (the one that points up or down), the molecule should be viewed from another angle so that the lowest priority group points away from the viewer. This can be achieved by doing 2 two-group switches or interchanges. If only 1 two-group interchange is done, the opposite configuration results. See the example given below.
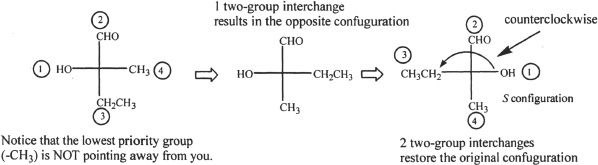
Example 19-1
Find the absolute configuration of the molecule shown below in terms of R-S notation.
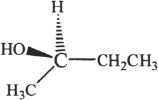
Solution:
First, we have to think about the order of priority of the substituents. The order is as follows:
OH > CH2CH3 > CH3 > H
We can simplify the structure by looking at the three substituents that determine the configuration. Let’s redraw them as shown below:
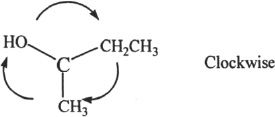
Since the direction of priority is clockwise, the configuration is R.
Example 19-2
Draw the L configuration of the compound shown in Example 19-1.
Solution:
For L configuration, the direction of priority should be counterclockwise. So the structure can be best represented as follows:
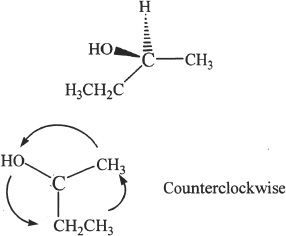
CHAPTER 19 PRACTICE QUESTIONS
1. What is the best term that can be used to express the relationship between the two structures shown below?
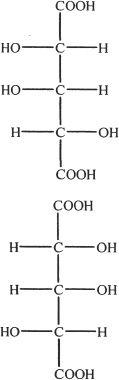
A. Diastereomers
B. Enantiomers
C. Mesocompounds
D. Anomers
2. What is the best term that can be used to express the relationship between the two structures shown below?
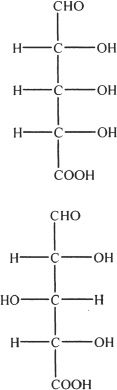
A. Diastereomers
B. Enantiomers
C. Identical compounds
D. None of the above
3. The structure shown below is that of 2,3,4-trihydroxy glutaric acid. Choose the correct enantiomer of this compound.
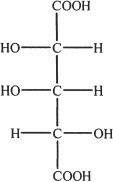
A. 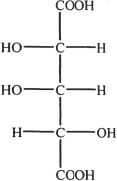
B. 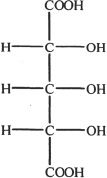
C. 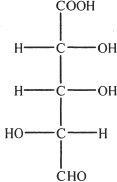
D. 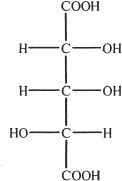
4. All are equivalent structures or representations of the compound given below, except:
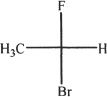
A. 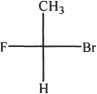
B. 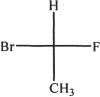
C. 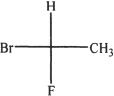
D. ![]()
5. What is the name of Compound X?
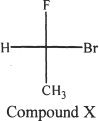
A. (S)-1-bromo-1-fluoromethane
B. (R)-1-bromo-1-fluoromethane
C. (S)-1-bromo-1-fluoroethane
D. (R)-1-bromo-1-fluoroethane
6. Which of the following are true?
I. Diastereomers are stereoisomers.
II. All stereoisomers are enantiomers.
III. All enantiomers are stereoisomers.
A. I & II only
B. I & III only
C. II & III only
D. I, II, & III
7. Saw horse representation of a compound is given below. Which of the following is true about this structure?
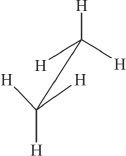
A. The compound has an eclipsed conformation.
B. The compound has a staggered conformation.
C. The compound has a cis-configuration.
D. None of the above