The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Alkyl Halides
A. INTRODUCTION
Alkyl halides are organic compounds with the general formula RX, where R denotes the alkyl group and X denotes the halogen.
B. NAMING OF ALKYL HALIDES
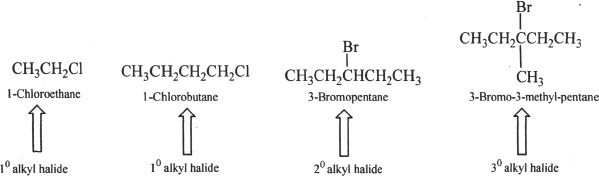
We can classify alkyl halides as primary (10), secondary (20), and tertiary (30) alkyl halides. The general formulas for these are as follows:

C. PROPERTIES OF ALKYL HALIDES
Alkyl halides have large dipole moments since the carbon-halogen bond is polar. They also have reasonably high boiling points because of the polarity and the high molecular weights.
![]()
Alkyl halides are generally insoluble in water. Density of alkyl halides increases as the atomic weight of the halogen present increases. The heavier the halogen is, the higher the density.

D. SYNTHESIS OF ALKYL HALIDES
From Alcohols
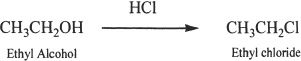
Alkyl halides can be prepared from alcohols. The following examples show some representative reactions. The conversion of an alcohol to an alkyl halide is an example of a nucleophilic substitution reaction.
Sample reaction 20-1
Sample reaction 20-2
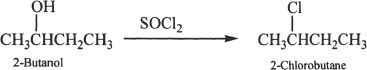
From Alkanes
Alkyl halides can be synthesized from alkanes. In this reaction, one of the alkane’s hydrogen atoms is substituted with a halogen atom. The reaction occurs by free radical mechanism.
Sample reaction 20-3

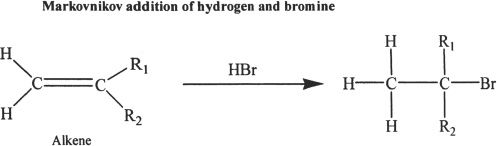
From Alkenes
Alkyl halides can be prepared from alkenes.
Sample reaction 20-4
![]()
Sample reaction 20-5
E. REACTIONS OF ALKYL HALIDES
Nucleophilic Substitution Reactions
In this section, we will discuss the two major substitution reactions — SN1 and SN2 reactions. In nucleophilic substitution reactions involving alkyl halides as the substrate, a Lewis base (nucleophile) substitutes the halogen present in the alkyl halide. We will discuss nucleophilic reactions in which alkyl halides react with nucleophiles. A general representation can be done as follows:
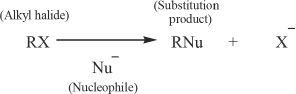
The SN2 Reaction
SN2 stands for bimolecular nucleophilic substitution reaction. Consider the reaction between methyl iodide and sodium hydroxide.
![]()
This reaction follows SN2 mechanism. Experimentally, it has been confirmed that the rate of this reaction depends on both the alkyl halide and the nucleophile (OH—) involved. The reaction rate is written as follows:
Rate = k [CH3I] [OH—]
The Mechanism of SN2 Reaction
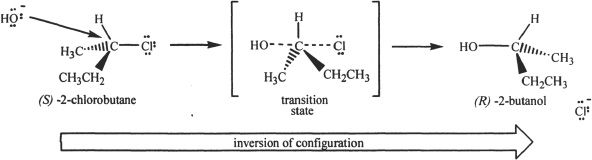
We will look at the mechanism involved in the reaction between methyl iodide and sodium hydroxide. The SN2 reaction proceeds via a five-coordinate transition state. This transition state has weak (the weak bonds are indicated by the dotted lines in the mechanism) carbon-iodine and carbon-oxygen bonds. Even though these two are weak bonds, the other three bonds involving the central carbon atom are complete bonds. As the leaving group detaches, there is an inversion of configuration (in chiral molecules) at the carbon where the leaving group was attached.
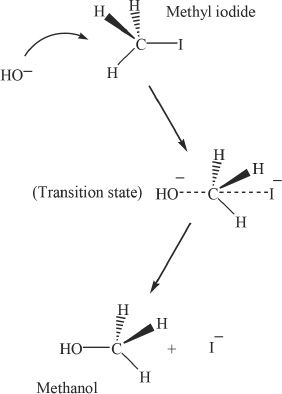
Figure 20-1 The mechanism of SN2 reaction
In SN2 reactions, the nucleophilic attack occurs opposite (backside attack) to the side where the leaving group is present. This type of nucleophilic approach is thermodynamically favored since the backside attack minimizes the electrostatic repulsions between the nucleophile and the leaving group involved. If substituents (especially bulky ones) are present on the carbon where the nucleophilic attack occurs, this can hinder the SN2 process. Hence, primary alkyl halides are the most reactive among alkyl halides with respect to SN2 reactions.
SN2 reactivity: Methyl > Primary > Secondary > Tertiary
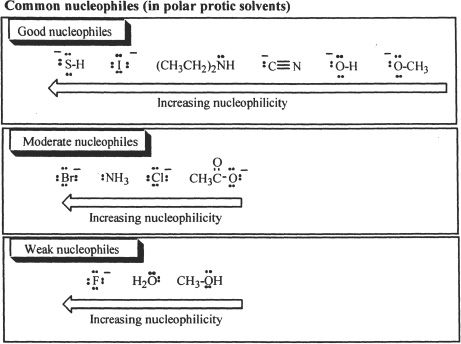
As mentioned earlier, the nucleophile and its concentration can influence the rate of SN2 reactions. So the rate is different for different nucleophiles. Nucleophilicity or the strength of a nucleophile depends on how efficiently it can make the leaving group leave from the alkyl halide or whatever the substrate is. Some generalizations can be made regarding nucleophiles. Along a period (in the periodic table), as basicity decreases, nucleophilicity decreases (e.g., F— < RO— < R2N— < R2C—). Along a group, as basicity decreases, nucleophilicity increases (e.g., F— < Cl— < Br— < I—).
Nucleophilicity can also be compared among species having the same nucleophilic atom. A negatively charged conjugate base of a neutral species (conjugate acid) is more nucleophilic than its corresponding neutral species. For example, HO— is a better nucleophile than H2O.
A leaving group plays an important role in both substitution and elimination reactions. A good leaving group has a weak, polarized carbon-leaving group (C-X) bond. It should be stable on its own once it leaves the substrate, regardless of whether it stays as an ion or a neutral species. Sometimes solvation helps a leaving group to achieve this. Halides are good leaving groups. The order of leaving group ability is F < Cl < Br < I. In general, less basic the species is, the better the leaving group. Other good leaving groups include mesylate and tosylate
The SN1 Reaction
SN1 stands for unimolecular nucleophilic substitution reaction. Let’s consider a typical SN1 reaction.
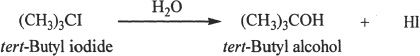
The rates of SN1 reactions depend only on the substrate (alkyl halide) concentration. The nucleophile does not influence the reaction rate of a typical SN1 reaction.
The reaction rate is represented as follows:
Rate = k [ (CH3)3CI ]
The Mechanism of SN1 Reaction
In this two-step reaction, the alkyl halide splits to form a carbocation intermediate and a halide ion. During the second step, the cation reacts with the nucleophile to form the final product. Since the carbocation formed is planar, the nucleophile can attack the electrophilic carbon from either side. Thus an SN1 reaction results in racemization.
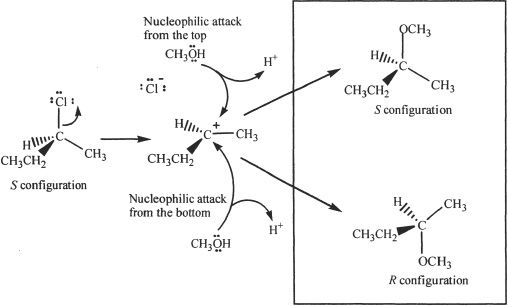
Figure 20-2 The mechanism of SN1 reaction
SN1 reactivity: Methyl < Primary < Secondary < Tertiary
SN2 reaction is favored when the alkyl halide involved is a primary or a secondary alkyl halide. SN1 reaction is favored when the alkyl halide involved is a tertiary or a secondary alkyl halide. In many cases, it is hard to predict the mode of reaction with secondary alkyl halides — it can either be SN1 or SN2, depending on certain other aspects such as the solvent used. The SN1 reaction being favored by tertiary halides is understandable, because of the carbocation intermediate that is formed during the SN1 process.
Polar solvents increase the rate of both types of substitution reactions. Polar solvents which have high dielectric constants can stabilize the transition state and this is highly useful in SN1 reactions. In SN2 reactions, the solvent effects are slightly different. Here what matters is whether the solvent is aprotic. Protic polar solvents such as water and carboxylic acids can undergo hydrogen bonding which in turn can interact with the nucleophile. This can decrease the rate of SN2 reactions. So it is better to use aprotic polar solvents when we are dealing with SN2 reactions. Dimethyl sulfoxide (DMSO) is a polar aprotic solvent.
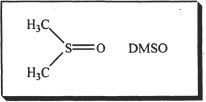
Quite often nucleophilic reactions compete with elimination reactions. Next, we will review elimination reactions.
Elimination reactions
There are two types of elimination reactions in general — E1 and E2 reactions. We will first consider an E2 reaction.
The E2 reaction
The E2 reaction mechanism can generally be represented as shown. In the mechanistic representation shown, B stands for the base and X stands for the halogen.
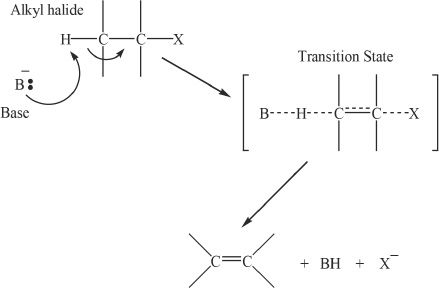
Figure 20-3 The mechanism of E2 reaction
The steps involved in an E2 reaction are the breaking of the carbon-hydrogen bond, the carbon double bond formation, and the breaking away of the carbon-halogen bond.
The rate of the E2 reaction is: Rate = k [RX] [base]
So the reaction rate depends on both the substrate (RX) and the base involved.
In an elimination reaction, the major product formed is the most stable alkene. Usually, the most stable alkene is the most substituted alkene. The increased stability of more highly substituted alkenes can be attributed to electronic effect.
The E1 Reaction
Study the mechanism of E1 reaction.
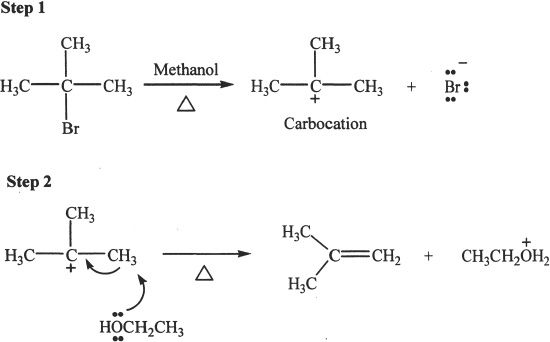
Figure 20-4 The mechanism of E1 reaction
In step (1), the alkyl halide forms (slow step) the carbocation and the halide ion. In step (2), the base abstracts the proton to form the product (alkene).
![]()
Substitution versus Elimination
Sometimes it is hard to predict the product of a reaction involving nucleophiles or bases. Why? Well, the reaction mechanisms are influenced by many factors. Different combination of factors result in different outcomes. Even though this is the case, let’s boil down some of the factors that we can rely on for reasonably judging the outcome of reactions. We will consider some conditions that favor substitution over elimination, and vice versa. The two key factors that we look for are the type of substrate that is undergoing the reaction, and the extent of nucleophilicity or basicity of the anion involved in the reaction.
1) Higher temperatures usually favor elimination over substitution. To be more precise, we can say that elimination is more favored than substitution reactions if the reaction occurs at a high temperature. The latter is more accurate because both types of reactions are favored by an increase in temperature, but elimination is more favored.
2) Strong bases guide or dictate elimination over substitution in most cases. In general, E2 type of elimination is favored under such conditions.
3) If there is less hindrance or less bulky substituents at the carbon where the leaving group is present, substitution (SN2) is favored over elimination (E2). Remember that in SN2 reactions, the transition intermediate is a species in which both the leaving group and the incoming nucleophile are attached to the carbon, where the substitution is taking place.
4) Alkyl halides (tertiary), because of their bulky substituents, mostly prefer elimination rather than substitution provided that a strong base is present. Mild bases can make substitution to predominate even in tertiary alkyl halides. Can you think of a reason why tertiary alkyl halides prefer to undergo elimination? The reason is steric hindrance to the nucleophilic approach.
Note: We have been discussing a number of reactions, both substitution and elimination in terms of alkyl halides. This surely doesn’t mean that only alkyl halides undergo these types of reactions.
Nucleophilic Substitution in Alkyl Halides - SN1 vs SN2 comparison
Table 20-1
SN1 |
SN1 |
|
Molecularity |
Unimolecular (First order) |
Bimolecular (Second order) |
Rate |
Rate = k [RX] |
Rate = k [RX] [Nu] |
Stereochemical aspect |
Racemization |
Inversion of configuration |
Rearrangement? |
Yes; Carbocation formation |
No; No carbocation formation |
Rate dependency |
Rate independent of nucleophile |
Rate dependent on nucleophile |
Solvent effect |
Best: Polar protic |
Best: Polar aprotic |
Reactivity |
RX<r2X<r3X</r</r |
R3X<r2X<rx< td=""></rx<></r |
Reactivity of alkyl halides |
RF<<rcl<rbr<ri< td=""></rcl<rbr<ri<> |
RF<<rcl<rbr<ri< td=""></rcl<rbr<ri<> |
CHAPTER 20 PRACTICE QUESTIONS
![]()
1. The compound shown above can be best described as a:
A. primary alkyl halide.
B. secondary alkyl halide.
C. tertiary alkyl halide.
D. primary alkenyl halide.
2. Pick out the compound with the highest boiling point.
A. 1-Chloroethane
B. 1,1-Dichloroethane
C. 1,1,1-Trichloroethane
D. Cannot be predicted
3. Alcohols can react with hydrogen halides to form alkyl halides. Which of the following alcohols is the most reactive toward hydrogen halides?
A. ![]()
B. 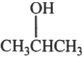
C. ![]()
D. ![]()
4. Which of the following is true regarding alkyl halides?
A. Alkyl halides have relatively very low boiling points, because of its polarity.
B. Alkyl fluorides have lower densities than those of comparable alkyl bromides.
C. Alkyl halides are completely soluble in water.
D. None of the above
Questions 5-9 are based on the following passage.
Passage 1
Alkyl halides undergo substitution reactions and a variety of other reactions as well. The type of reaction that occurs depends on a variety of factors. The structural integrity of the alkyl group to which the halogen is attached plays an important role in determining the type of product that is likely to result. A reaction involving t-butyl bromide is given below:
![]()
5. Which of the following best represents the increasing rate of bimolecular substitution reaction for the following alkyl halides?
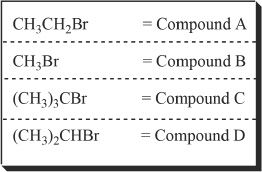
A. A < B < C < D
B. C < D < B < A
C. C < D < A < B
D. B < A < D < C
6. Which of the following is true regarding substitution reactions?
A. Tertiary alkyl halides always undergo substitution by SN2 mechanism.
B. Only alkyl halides can undergo substitution reaction by SN2 mechanism.
C. SN2 reactions involve the formation of a carbocation intermediate.
D. None of the above
7. Consider the reaction given in the passage. Which of the following compound is produced as a result of that particular reaction?
A. 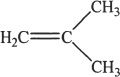
B. ![]()
C. ![]()
D. ![]()
8. Which of the following is characteristic of a typical SN2 reaction?
A. Rate = k [alkyl halide]
B. Inversion of configuration
C. Carbocation intermediate
D. Unimolecular mechanism
9. Which of the following is the most reactive by substitution mechanism?
A. CH3I
B. CH3F
C. CH3Cl
D. All are equally reactive.