The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Alcohols
A. INTRODUCTION
Alcohols have the general formula ROH.
Some common examples of alcohols:
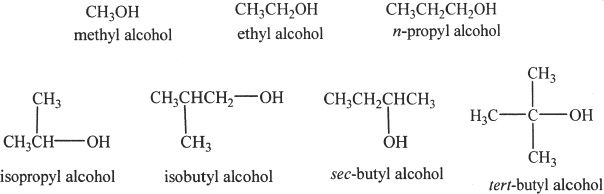
B. PROPERTIES OF ALCOHOLS
Boiling Point
One of the most important properties of alcohols is their ability to form hydrogen bonds. Since alcohols can form hydrogen bonds, they have very high boiling points. Consider the following comparative analysis to clarify this idea.
The boiling point of ethane (C2H6) is — 88.7 0 C.
The boiling point of propane (C3H8) is — 42.2 0 C.
The boiling point of ethanol (C2H5OH) is 78 0 C.
Note the drastic difference in their boiling points.
By the above comparison, it is clear that some extra forces (here mainly H bonds) are working to increase the boiling points of alcohols.
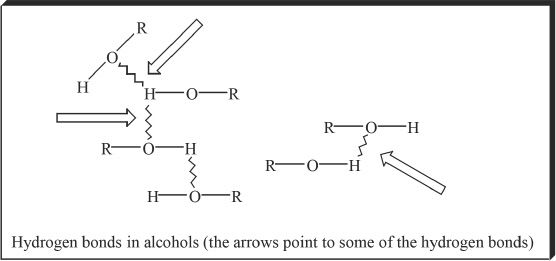
Figure 21-1
Solubility in H2O
Low-molecular weight alcohols are soluble in water. The hydrogen bond formation plays a key role in the solubility of alcohols in water. In other words, hydrogen bonds enhance the solubility of alcohols in H2O. As the number of carbons increases, the solubility of alcohols decreases as they become more and more similar to hydrocarbons with longer and longer nonpolar hydrocarbon chains.
Acidity of Alcohols
Alcohols are weakly acidic. In fact, they are weaker in acidity than water. Alcohols have Ka values around 10—17.
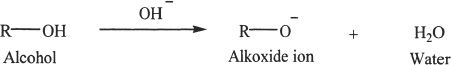
Alcohols give off the acidic hydrogen to form alkoxide ions. Alkoxide ion is the conjugate base of alcohols and they are strong bases.
Among alcohols, primary alcohols are the most acidic, and tertiary alcohols are the least acidic.
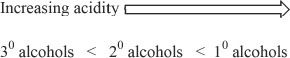
C. SYNTHESIS OF ALCOHOLS
Acid-Catalyzed Reaction
Sample reaction 21-1
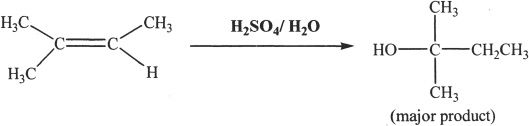
Alkenes react with aqueous acidic solutions to form alcohols. The reaction intermediate is a carbocation. Hence, there is a possibility of rearrangement of the intermediates in such acid-catalyzed reactions. The reaction follows the Markovnikov’s rule. Keep in mind that a tertiary carbocation is more stable than a secondary carbocation, which in turn is more stable than a primary carbocation.
Hydroboration-Oxidation
Sample reaction 21-2
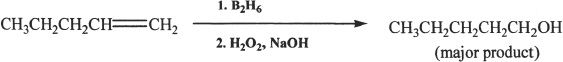
Oxidation followed by hydroboration of alkene results in alcohols. The reaction occurs in an anti-Markovnikov fashion. Notice that hydrogen, instead of attaching to the carbon with the highest number of hydrogens, attaches to the carbon with the least number of hydrogens.
Oxymercuration-Demercuration
Sample reaction 21-3
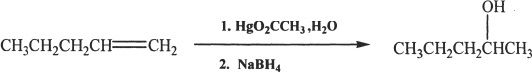
Alkenes can be converted to alcohols by oxymercuration-demercuration. The addition of H and OH is in accordance with the Markovnikov’s rule. There is no rearrangement in this reaction.
From Epoxides
Preparation of an alcohol from an epoxide is shown below. The epoxide (ethylene oxide) ring opens when the nucleophile attacks the carbon-oxygen bond. Note the fact that the nucleophilic carbon is supplied by the Grignard reagent (methyl magnesium bromide).
Sample reaction 21-4
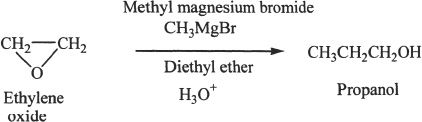
Alcohols can also be produced from aldehydes and ketones via Grignard reagents. Other methods to synthesize alcohols include the reduction of aldehydes and ketones. These reactions will be discussed in Chapter 22.
D. REACTIONS OF ALCOHOLS
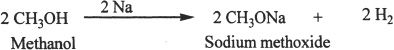
Formation of Alkoxides
Alcohols when treated with Group I metals like sodium and potassium result in alkoxides.
Sample reaction 21-5
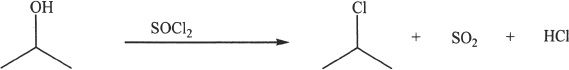
Formation of Alkyl Halides
Sample reaction 21-6

Sample reaction 21-7
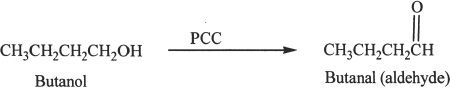
Oxidation of Alcohols
Primary alcohols can be oxidized to aldehydes by reagents like pyridinium dichromate (PDC), and pyridinium chlorochromate (PCC).
Sample reaction 21-8
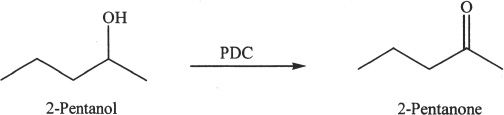
Secondary alcohols can also be oxidized. The product is a ketone.
Sample reaction 21-9
Since tertiary alcohols do not have a hydrogen atom in the carbon carrying the hydroxyl group, they cannot be easily oxidized.
Strong oxidizing agents can oxidize alcohols to carboxylic acids. We can use potassium permanganate or chromic acid to do this conversion.
Sample reaction 21-10
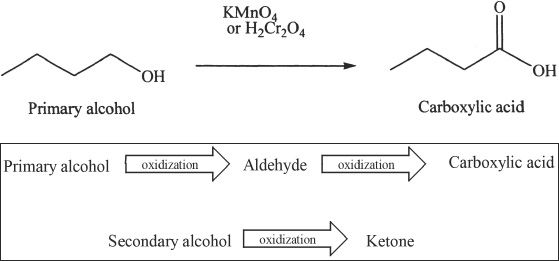
Acid-Catalyzed Dehydration
Alcohols undergo acid-catalyzed dehydration reactions to form alkenes. The reaction is accomplished by the formation of a carbocation intermediate. Thus there is the possibility of rearrangement in the process. First, we will take a look at the mechanism of this reaction.
Mechanism of Acid-Catalyzed Dehydration of Alcohols
Sample reaction 21-11
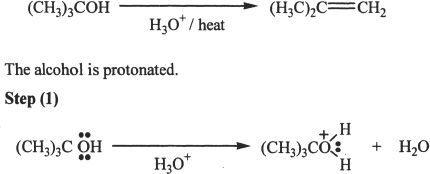
The formation of carbocation.
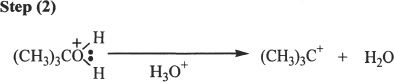
The formation of double bond.
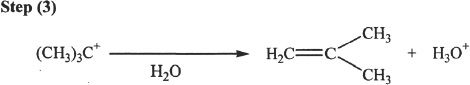
Rearrangement — An Example
Organic chemists have conducted experiments to understand the formation of multiple products in acid-catalyzed reactions such as that of 3,3-dimethyl-2-butanol. Let’s consider the acid-catalyzed dehydration reaction of 3,3-dimethyl-2-butanol and explore the possibility of rearrangement.
![]()
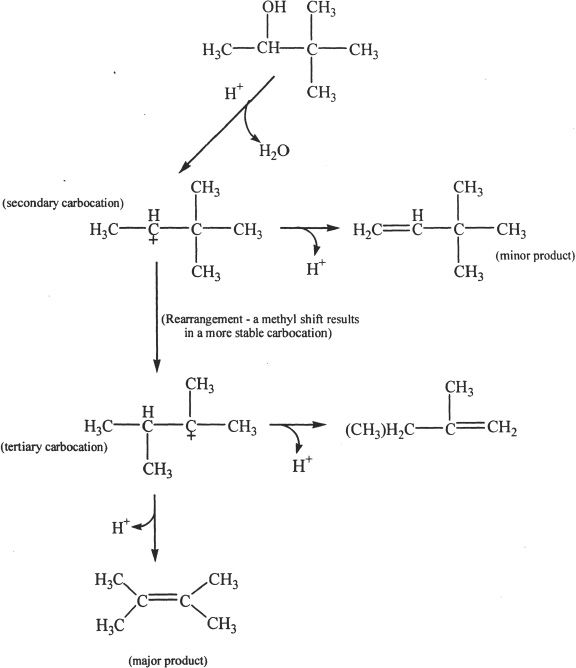
Figure 21-2
The major product formed in this reaction is 2,3-Dimethyl-1-butene. Notice (Figure 21-2) the rearrangement that occurs in this reaction. The rearrangement results in a more stable carbocation (A tertiary carbocation is more stable than a secondary carbocation).
CHAPTER 21 PRACTICE QUESTIONS
1. The higher boiling points exhibited by alcohols with respect to comparable alkanes and ethers are primarily due to:
A. ionic bonds.
B. covalent bonds.
C. hydrogen bonds.
D. none of the above.
2. The conjugate base of alcohols are generally called:
A. alkyl ions.
B. alcoholic ions.
C. alkoxide ions.
D. vinyl ions.
3. Which of the following is a possible way of making alcohols?
A. Hydroboration-oxidation of alkenes
B. Acid catalyzed hydration of alkenes
C. Oxymercuration-demercuration of alkenes
D. All the above
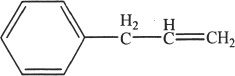
4. An organic chemist synthesized and isolated the above represented compound. If this compound undergoes oxymercuration-demercuration reaction, the major product is most likely:
A. 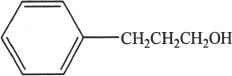
B. 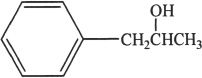
C. 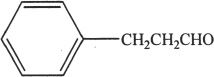
D. 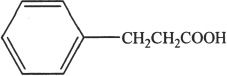
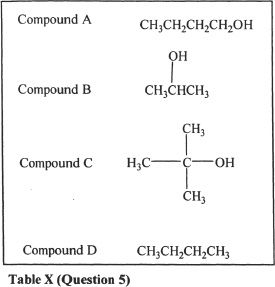
5. From Table X, the most acidic compound is:
A. Compound A
B. Compound B
C. Compound C
D. Compound D
6. Direct oxidation of which of the following can result in a carboxylic acid?
A. Secondary alcohol
B. Tertiary alcohol
C. Primary alcohol
D. All the above
7. Choose the correct product formed as a result of the reduction of a ketone.
A. A primary alcohol
B. A secondary alcohol
C. A tertiary alcohol
D. None of the above
8. The major product of the following reaction is:
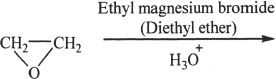
A. a four-carbon ketone.
B. a four-carbon halohydrin.
C. a two-carbon aldehyde.
D. a four-carbon alcohol.
9. Identify Compound B from the sequence of reactions.
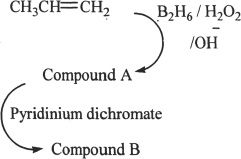
A. Propanol
B. Propanal
C. Acetone
D. Formaldehyde
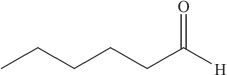
10. Reduction of the organic compound shown above results in:
A. a ketone.
B. a primary alcohol.
C. a carboxylic acid.
D. an ester.
Questions 11-15 are based on the following passage.
Passage 1
Alcohols are of tremendous use for the synthesis of a wide variety of organic compounds. They also have some very unique properties. Alcohols are in fact slightly weaker acids than water, and have pKa values between 16 and 18. An alcohol can loose its acidic hydrogen to form an alkoxide ion.
11. The alcohol shown below is:
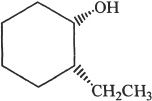
A. cis-2-ethylcyclohexanol.
B. cis-2-ethylcycloctanol.
C. cis-1-ethyl-2-hydroxycyclopentane.
D. trans-2-ethylcyclohexanol.
12. Which of the following is the most acidic?
A. (CH3)3COH
B. NH3
C. (CH3)2CHOH
D. CH3OH
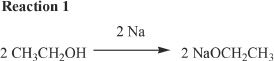
13. In Reaction 1, predict the identity of the other product, if any.
A. H2O
B. O2
C. H2
D. No other products are produced.
14. Which of the following is the most reactive alcohol toward sodium?
A. ![]()
B. ![]()
C. 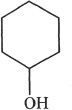
D. 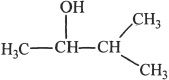
15. Which of the following can be used to make propanol directly by oxidation?
A. ![]()
B. ![]()
C. ![]()
D. ![]()