The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Aldehydes and Ketones
A. INTRODUCTION
Aldehydes and ketones are compounds containing the acyl group. The carbonyl group present in them is polar because of the electronegativity of oxygen. It is noteworthy to mention that the carbonyl group in aldehydes and ketones are very reactive.
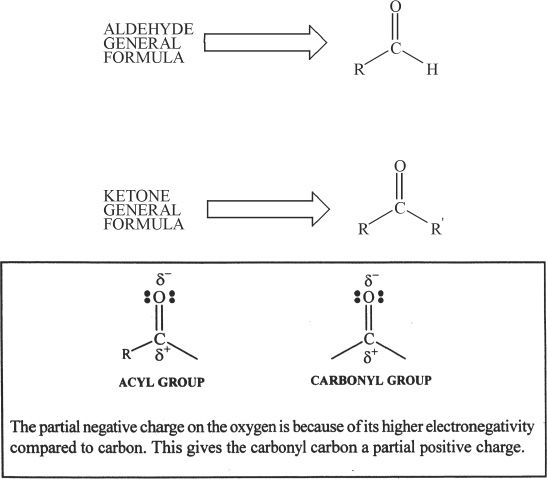
B. SOME ALDEHYDES AND KETONES
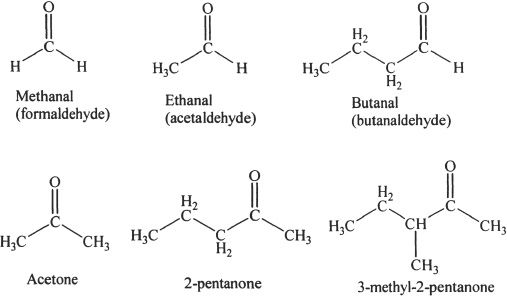
C. PROPERTIES OF ALDEHYDES AND KETONES
The carbonyl group present in aldehydes and ketones make them polar in nature. Both aldehydes and ketones have higher boiling points than comparable hydrocarbons. Aldehydes and ketones do not form hydrogen bonds themselves. Hence, they have lower boiling and melting points compared to alcohols with comparable molecular weights. Finally, aldehydes and ketones have higher solubility in water than hydrocarbons, but less solubility than alcohols. Even though aldehydes and ketones cannot undergo hydrogen bonding themselves, they can undergo hydrogen bonding with water molecules.
D. SYNTHESIS OF ALDEHYDES AND KETONES
From Alcohols
Aldehydes can be prepared by the oxidation of primary alcohols. Oxidizing agents such as pyridinium chlorochromate (PCC) or pyridinium dichromate (PDC) can be used for such conversions.
Sample reaction 22-1
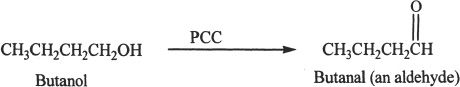
Oxidation of secondary alcohols results in ketones.
Sample reaction 22-2

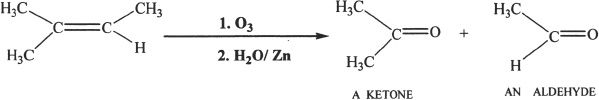
From Alkenes by Ozonolysis
Aldehydes and ketones can be prepared by the ozonolysis of alkenes.
Sample reaction 22-3
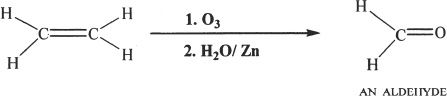
Sample reaction 22-4
E. NUCLEOPHILIC ADDITION TO THE CARBONYL GROUP
The carbon of the carbonyl group is an electrophile. Thus it is susceptible to nucleophilic attack. During most addition reactions of alkenes, it is an electrophile that attacks the pi system. But in an addition reaction involving a carbonyl group, we see that the attack on the carbonyl carbon is made by a nucleophile. Think about why this is the case? One reason for this is the polar nature (due to electronegativity of the oxygen atom) of the carbonyl group. Furthermore, the resonance capability of the carbonyl group also enhances this type of reactive property. A generalized nucleophilic attack is shown below in Figure 22-1.
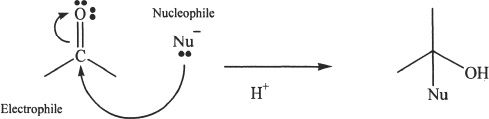
Figure 22-1
F. REACTIONS OF ALDEHYDES AND KETONES
Formation of Carboxylic Acids
Aldehydes can be oxidized to carboxylic acids using oxidizing agents such as potassium permanganate.
Sample reaction 22-5
![]()
Sample reaction 22-6
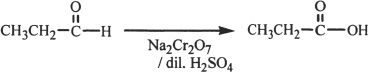
Sample reaction 22-7
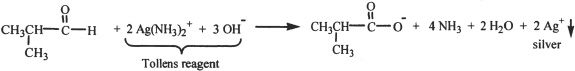
With Grignard Reagents
Aldehydes and ketones react with Grignard reagents to form alcohols.
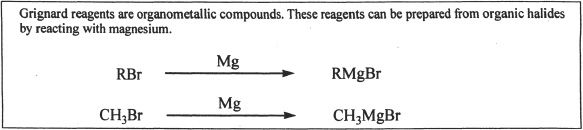
Formaldehyde reacts with Grignard reagents to form primary alcohols.
Sample reaction 22-8
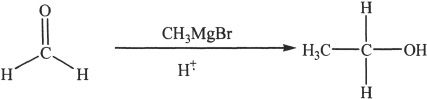
Other aldehydes react with Grignard reagents to form secondary alcohols.
Sample reaction 22-9
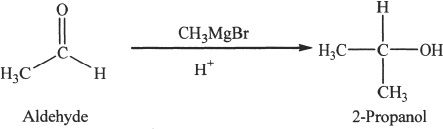
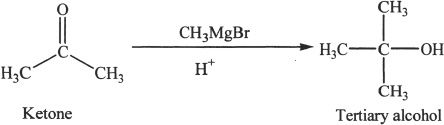
Ketones give tertiary alcohols.
Sample reaction 22-10
G. ALDOL ADDITION AND CONDENSATION REACTIONS
In aldol addition reactions, a carbon-carbon bond is formed between the carbonyl carbon of one aldehyde molecule and the α-carbon of another aldehyde molecule. The product is a β-hydroxy aldehyde. After the nucleophilic addition of the enolate ion to the carbonyl group, removal of water of the β-hydroxy aldehyde can occur resulting in an α,β-unsaturated aldehyde. Study Sample reactions 22-11 and 22-12.
Sample reaction 22-11
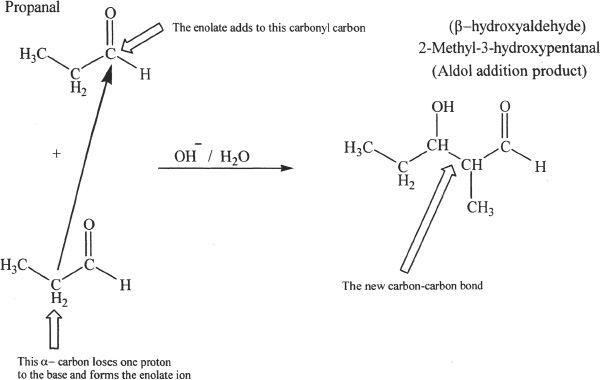
Sample reaction 22-12
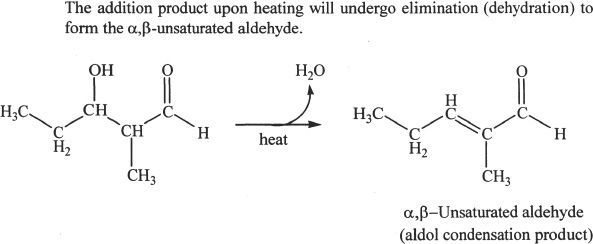
H. REDUCTION REACTIONS OF ALDEHYDES AND KETONES
Conversion to Alcohols
Aldehydes and ketones can be reduced to alcohols using reducing agents such as lithium aluminum hydride (LiAlH4), and sodium borohydride (NaBH4).
Sample reaction 22-13
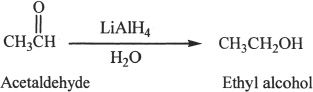
Sample reaction 22-14
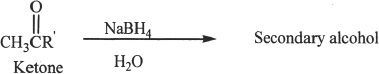
Conversion to Hydrocarbons
The carbonyl groups of aldehydes and ketones can be converted to methylene groups by Clemmenson and Wolf-Kishner reduction reactions.
Sample reaction 22-15
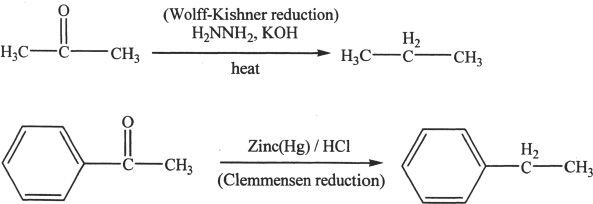
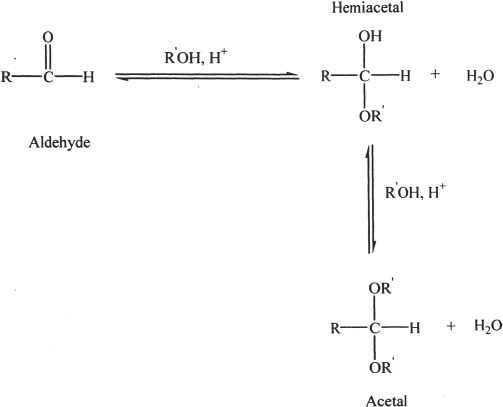
I. FORMATION OF ACETALS
Aldehydes can undergo reactions with alcohols under acidic conditions. The product of this nucleophilic addition reaction is called hemiacetal. Hemiacetal is formed by the nucleophilic addition of alcohol to the carbonyl carbon. Hemiacetal can react with one more mole of alcohol to form an acetal.
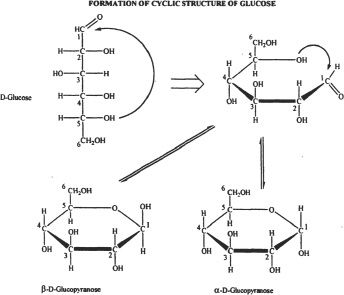
In monosaccharides (e.g., glucose, fructose), the cyclic ring structure is formed by the intramolecular reaction between the carbonyl and the hydroxyl groups within the same molecule. Glucose (an aldohexose) forms a cyclic hemiacetal by the intramolecular reaction between the aldehyde group of carbon 1 and the hydroxyl group of carbon 5.
CHAPTER 22 PRACTICE QUESTIONS
1. Propanal is treated with ethyl magnesium halide (with diethyl ether & H3O+). Which of the following is true regarding this reaction?
A. A five-carbon ether is the major product.
B. A five-carbon secondary alcohol is the major product.
C. A five-carbon ketone is the major product.
D. None of the above are true.
2. The structure shown below represents:
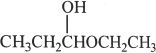
A. a ketal.
B. a hemiacetal.
C. an acetal.
D. a hemiketal.
3. The Baeyer-Villiger reaction is a very useful synthesis reaction. An example is the reaction of acetone with peroxyacetic acid to give an ester and a carboxylic acid. This reaction can be generalized as:
A. a reduction reaction.
B. an oxidation reaction.
C. a hydrolysis reaction.
D. an isomerization reaction.
4. Predict the aldol addition product of the structure shown below:
A. 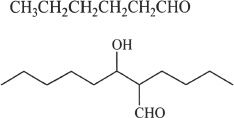
B. ![]()
C. ![]()
D. 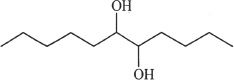
5. Which of the following carbons indicated by arrows has the most acidic protons?
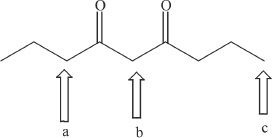
A. a
B. b
C. c
D. No acidic protons are present, since ketones cannot be acidic.
6. The structure represented below can be most appropriately called an:
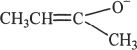
A. alkenyl ion.
B. acyl ion.
C. alkoxide cation.
D. enolate ion.
7. Predict the aldol condensation product (major product) of the following compound.
A. 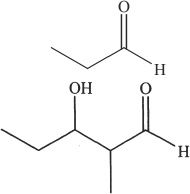
B. 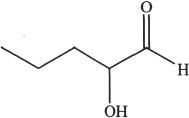
C. 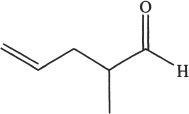
D. 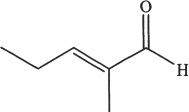
8. Which of the following is the conjugate base of a ketone?
A. 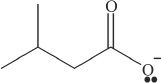
B. 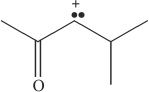
C. 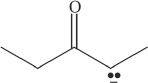
D. 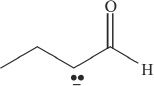
9. A student is trying to prove the identity of a compound that she synthesized. She knows that the compound is a methyl ketone. Which of the following qualitative tests can be used to prove the identity of the synthesized compound as a methyl ketone?
A. Ignition test
B. Potassium permanganate test
C. Beilstein test
D. Iodoform test
10. Chromic acid test can be used for the identification of:
A. aldehydes and ketones.
B. aldehydes only.
C. ketones only.
D. carboxylic acids only.
Questions 11-16 are based on the following passage.
Passage 1
Students of an organic chemistry class conducted several experiments with aldehydes and ketones. Some of the reactions are summarized below:
Experiment 1
Acetophenone was reacted with Reagent
A. The product was ethyl benzene.
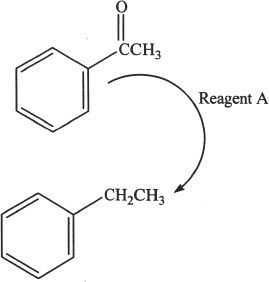
Experiment 2
Propanal underwent acid-catalyzed reaction with alcohol.
![]()
Experiment 3
![]()
Experiment 3 involved merely the analysis of a conversion. Students were asked to discuss a method to accomplish this synthesis reaction.
Experiment 4
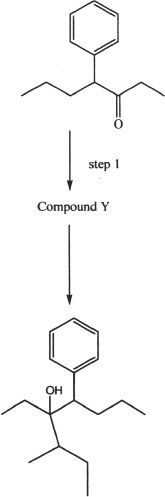
11. Which of the following best represents the general name of the product formed in Experiment 2?
A. Hemiacetal
B. Acetal
C. Ketal
D. Hemiacetal
12. The conversion presented in Experiment 1 is:
A. an oxidation reaction.
B. a reduction reaction.
C. a dehydrohalogenation reaction.
D. a dehydrogenation reaction.
13. Which of the following Grignard reagents will serve the purpose for the reaction in Experiment 4?
A. ![]()
B. ![]()
C. ![]()
D. 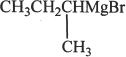
14. In Experiment 3, which of the following is a possible scenario?
A. Reaction is possible, because propanal can be directly oxidized to form the product indicated.
B. Reaction is not possible, because you cannot convert an aldehyde to a secondary alcohol.
C. Reaction is possible, if the reactant is treated with an appropriate Grignard reagent followed by hydrolysis.
D. Reaction is not possible, because aldehyde carbonyls are nucleophilic and are thus unreactive.
15. Which of the following is not an organometallic compound?
A. ![]()
B. ![]()
C. 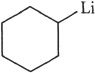
D. ![]()
16. In our body, toxic chemicals are metabolized in the liver. One such process involves the production of acetaldehyde from ethanol. This conversion is best described as:
![]()
A. a dehydrogenation reaction.
B. a hydrogenation reaction.
C. an oxidation reaction.
D. a reduction reaction.