The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Periodic Table
A. INTRODUCTION
The periodic table is a systematic representation of elements in a particular order. From the periodic table, we can gather a tremendous amount of information about the characteristics of an element. In this chapter, we will look at the trends and other important aspects of the periodic table.
The properties of elements are periodic functions of their atomic numbers.
B. THE PERIODIC TABLE
The vertical columns of elements represented in the periodic table are called groups, and the horizontal rows are called periods. There are seven periods in the periodic table. The groups are usually designated by roman numerals followed by the letter A or B as shown in the periodic table.
The groups IA through VIIA are called the representative elements. These elements have either s or p orbital valence electrons. The last group in the periodic table is the noble gas group otherwise known as the zero group. The groups ranging from IB through VIII are called transition metals, and finally the metals from lanthanum through hafnium and metals from actinium onward are called the inner transition metals.
We will discuss some of these groups.
Group IA
The Group IA contains hydrogen, lithium, sodium, potassium, rubidium, cesium, and francium. The Group IA elements are also known as alkali metals, with the exception of hydrogen which is not a metal. Alkali metals are very reactive.
All of them react with water to form alkaline solutions.
![]()
The reactivity of alkali metals to water increases from top to bottom of the periodic table. For example, potassium reacts much more rapidly than lithium. They can also form oxides (For example, lithium can form oxides such as Li2O.) and a variety of other compounds, since they are highly reactive. Alkali metals are good electrical and thermal conductors. All of them have one valence electron in their outer most shell, which is in the s orbital in the ground state. The Group IA elements usually exhibit an oxidation state of +1. They have a valence shell configuration of ns1.
Group IIA
The Group IIA elements are called alkaline earth metals. The alkaline earth metals consist of beryllium, magnesium, calcium, strontium, barium, and radium. Their oxides are basic. They have a valence shell configuration of ns2, and exhibit an oxidation state of +2. These elements are not as reactive as alkali metals.
Metallic character decreases from left to right along a period, and increases from top to bottom of a group.
Group IIIA
The Group IIIA elements consist of boron, aluminum, gallium, indium, and thallium. They have a valence shell configuration of ns2np1. They usually have oxidation states of +1 and +3.
Group IVA
The Group IVA is the carbon family. Carbon is the most versatile element, and thus it has its own separate subject. Yes, you guessed right - organic chemistry. Carbon can exist in many different forms by itself such as graphite and diamond. These forms of carbon are very contrasting in the sense that graphite is relatively soft whereas diamond is very hard. The Group IVA elements have a valence shell configuration of ns2np2.
The carbon family consists of carbon, silicon, germanium, tin, and lead. All these form oxides which look like CO2 (e.g., SiO2, PbO2). They also form monoxides. As medical enthusiasts, you probably have heard of carbon monoxide, and its harmful effects. CO is a colorless and odorless gas, and it has even higher affinity for hemoglobin than oxygen in the red blood cells.
Group VA
The Group VA is the nitrogen family. The group consists of nitrogen, phosphorus, arsenic, antimony, and bismuth. Nitrogen is a diatomic, colorless, and odorless gas, and is not a very reactive element. The Group VA elements have a valence shell configuration of ns2np3.
Group VIA
The Group VIA elements are oxygen, sulfur, selenium, tellurium, and polonium. They have a valence shell configuration of ns2np4. Oxygen (O2) is a diatomic gas, and it also exists in an allotropic form called ozone (O3). Sulfur forms acidic oxides (e.g., SO2, SO3).
Group VIIA
The Group VIIA is more commonly known as the halogen family of elements. They are fluorine, chlorine, bromine, iodine, and astatine. They have an outer configuration of ns2np5. Halogens are highly reactive nonmetals, and form diatomic molecules. Halogens form hydrogen halides which are very acidic. These hydrogen halides can dissolve in water to form aqueous acids (e.g., HCl).
Fluorine |
- |
yellow gas |
Chlorine |
- |
greenish-yellow gas |
Bromine |
- |
reddish brown liquid |
Iodine |
- |
dark colored solid |
Group VIIIA
The elements of the Group VIIIA, otherwise known as noble gases are extremely unreactive. They are found as non-combined forms in nature. Because of this, they are called inert gases. They have an outer configuration of ns2np6.
C. PERIODIC TRENDS — ONE BY ONE
In this section, we will discuss the periodic properties and trends. It is very important from the MCAT point of view to understand the trends of the periodic table.
Atomic Size
As mentioned earlier, the periodic table is very versatile. The periodic table can give you the relative atomic sizes of atoms, and elemental ions. The two trends regarding the atomic radii are given below.
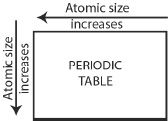
1. From left to right along a period, the atomic radius decreases, as the atomic number increases.
2. Along a group from top to bottom, the atomic radius increases.
One reason for such a trend is attributed to the principal quantum number. As the principal quantum number increases, the size of the orbital increases. Another reason for this trend is attributed to the nuclear shielding by the electron cloud that is between the nucleus and the outermost shells, thereby decreasing the influence of the effective nuclear charge.
Problem 4-1
Arrange the following elements in terms of increasing atomic radius: Mg, K, Cl, Ba
A) Cl, Mg, K, Cs
B) Cl, K, Mg, Cs
C) Cs, K, Mg, Cl
D) Mg, K, Cl, Ba
The answer is choice A. Mg and Cl are in the same period (Period 3). But Cl is in Group VIIA and Mg is in Group IIA. So Mg is larger than Cl. The next is K which is in period 4, and thus bigger than both Mg and Cl. Finally, Cs which is in period 6 has the largest atomic radius. So in the increasing order of atomic size is: Cl<mg<k<cs.< p=""></mg<k<cs.<>
Ionic Radius
Often you will get questions on arranging ions and atoms according to their sizes. Some of the trends that you should keep in mind regarding ionic radii are listed below:
1. Negatively charged ions have bigger ionic radii than the corresponding neutral atoms.
2. Positively charged ions have smaller ionic radii than the corresponding neutral atoms.
Ionization Energy (IE)
Ionization energy is the minimum amount of energy required to remove an electron from an atom. The amount of energy required to remove the first electron is called the first ionization energy (IE1). The second ionization energy (IE2) refers to the amount of energy required to remove the second electron. The second ionization energy is always greater than the first ionization energy, since it is more difficult to remove a second electron from an already positive ion, compared to the removal from an electrically neutral atom. The third ionization energy is greater than the second ionization energy, and so on.
First IE < Second IE < Third IE < Fourth IE < Fifth IE < Sixth IE < …
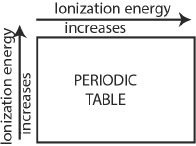
The general trend of ionization energy is summarized as follows:
1. Generally, along a period from left to right, the ionization energy increases with increasing atomic number.
2. The ionization energy decreases from top to bottom along a group as the atomic size increases.
Electron Affinity
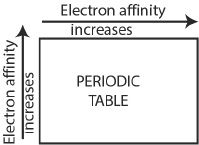
Electron affinity is the change in amount (energy either released or absorbed) of energy for the process of adding an electron to an atom (neutral) in its gaseous state, resulting in an ion of —1 charge. The general trend of electron affinity is given below:
The electron affinity increases or in other words, the negativeness of the electron affinity increases from left to right along a period.
Electronegativity
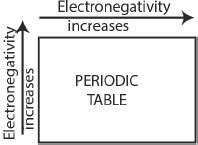
The relative tendency of an atom to attract the bonding electrons to itself is called electronegativity. The popularly used electronegativity scale is based on a system called Pauling’s scale, according to which fluorine (the most electronegative element) has an electronegativity value of 4.0. Nonmetals are the most electronegative elements. The general trend of the electronegativity is as follows:
1. Generally, the electronegativity increases from left to right along a period.
2. The electronegativity decreases down a group from top to bottom.
CHAPTER 4 PRACTICE QUESTIONS
1. Sulfur belongs to the classification of elements called the:
A. inner transition elements.
B. representative elements.
C. transition elements.
D. alkali metals.
2. Which of the following is an alkaline earth metal?
A. Na
B. Mn
C. Sr
D. Fe
3. Which of the following elements has the lowest electronegativity?
A. Rb
B. F
C. S
D. Ba
4. Which of the following belongs to the f block in the periodic table?
A. Sc
B. U
C. Si
D. Pd
5. Which of the following is not diatomic?
A. Oxygen
B. Nitrogen
C. Neon
D. Chlorine
6. Choose the atom that has the biggest atomic radius.
A. Al
B. Cl
C. Ge
D. Rb
7. Which of the following represents the correct arrangement of atoms according to their atomic radii?
A. F < B < Ca < Sr
B. B < F < Sr < Ca
C. Sr < Ca < B < F
D. F < B < Ca < Sr
8. Choose the true statement regarding the periodic trends.
A. Along a period from left to right, atomic radius increases.
B. Along a period from left to right, ionization energy decreases.
C. Electronegativity of elements increases from right to left along a period.
D. None of the above.
9. Which of the following is the most acidic?
A. HF
B. HBr
C. HCl
D. HI
10. The outer electronic configuration ns2 np2 belongs to which of the following groups?
A. II A
B. IV A
C. IV B
D. II B