The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Chemical Bonding
A. INTRODUCTION
MCAT students are expected to know the electronic structures of atoms and apply this knowledge to the formation of bonds and other related aspects. This chapter is devoted to the review of chemical bonding and related aspects such as ionic character and polarity.
What are Chemical Bonds?
Chemical bonds are strong attractive forces that enable atoms or groups of atoms to hold together. The two major categories of chemical bonds are ionic and covalent bonds. In this chapter, we will discuss ionic bonds, covalent bonds, and other atomic and molecular interactions.
B. IONIC BOND
The major force behind the formation of an ionic bond is the electrostatic attractive force that exists between negative and positive ions. It is formed by the transfer of one or more electrons from one atom to another. The atom that donates the electrons becomes positive (cation), and the counterpart atom that receives those electrons becomes negative (anion). The attractive force between two oppositely charged ions or species holds the atoms together in an ionic bond.
In an ionic compound, any ion can attract not only the pairing ion or group, but it can also attract neighboring oppositely charged ions, resulting in strong ionic solids.
Now let us take a look at an example to understand this better. The sodium fluoride (NaF) molecule is a result of ionic bond formation between sodium and fluoride ions. Together with this, you should also try to understand Lewis dot structures. Lewis structure will be discussed in detail later in this chapter.
Lewis Electron-Dot Formulas: Lewis electron-dot formulas are diagrammatic representations of the atoms involved and their valence electrons. The valence electrons are usually represented as dots around the elemental symbol.
Formation of the Ionic Bond in NaF

In the formation of the ionic bond, the sodium atom loses the electron from its 3s subshell, thereby becoming Na+.
![]()
On the other hand, the fluorine atom takes the electron that is being lost from the sodium atom to form a (F-) fluoride ion.
![]()
These resulting ions are oppositely charged and therefore have electrostatic attractive forces between them, resulting in the formation of the ionic bond.

The attractive energy in an ionic bond can be expressed in terms of Coulombic energy, according to Coulomb’s law. Imagine this by considering that the ions are spherical and are separated by particular distances. The attractive energy can be expressed as follows:

Here, k (= 9 x 109 J.m/C2) is a constant, q1 and q2 are the charges, and r is the distance between the nuclei of the two ionic entities involved in the bonding.
C. COVALENT BOND
A covalent bond is formed as a result of the sharing of a pair of electrons between atoms. Covalent bonds result when the difference in electronegativities between the bonding atoms is very small. Though the intramolecular bonds of covalent compounds are significant, the intermolecular forces are relatively weak. Because of this, covalent compounds have relatively lower boiling and melting points when compared to ionic compounds.
Covalent Bond Formation
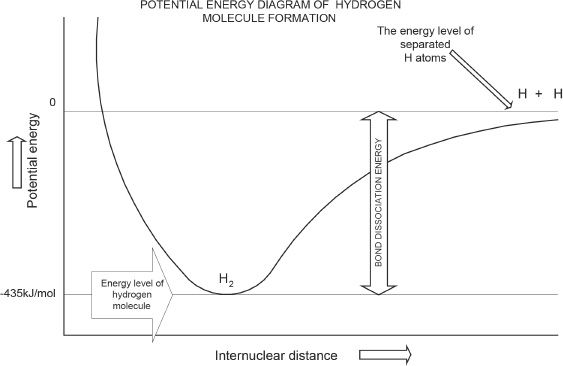
Figure 5-1
In this section, we will look at the covalent bond formation in the hydrogen molecule (H2). The hydrogen molecule is diatomic. The hydrogen atom has an electronic configuration of 1s1. The formation can be expressed in terms of Lewis formula as follows:
![]()
After the bond is formed, the electrons are shared by both hydrogen atoms, as expected in a covalent bond. This 1s overlap makes the configuration of hydrogen atom the same as that of helium (1s2). Another aspect that you should understand is that the total potential energy (see Figure 5-1) of the hydrogen molecule is lower than that of the hydrogen atoms in their separate forms. The potential energy diagram of hydrogen molecule formation should make this clear.
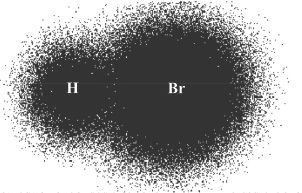
Figure 5-2
The distribution of bonded electrons in the HBr molecule is shown above (Figure 5-2). In this bond formation, hydrogen shares its electron with bromine, whereas bromine gives the one electron that is needed for hydrogen to complete its shell. Bromine atom has 7 electrons in its outermost shell, and it requires 1 more to complete its octet. Octet is the state in which an atom has 8 electrons in its outermost shell. Now, since hydrogen has 2 electrons, and bromine has eight electrons in its valence shell, they are both satisfied in terms of their stability, the stability being facilitated by the formation of the covalent bond.
D. COORDINATE COVALENT BOND
In coordinate covalent bonds, the same aspect of the sharing of electrons exists, just as in simple covalent bonds, but the difference is that both shared electrons are supplied by the same atom.
Coordinate covalent bond is formed when the two electrons that are shared in the formation of the bond are donated by one group or atom involved in the bond.
An example of coordinate covalent bond is seen in the formation of ammonium ion (NH4+).
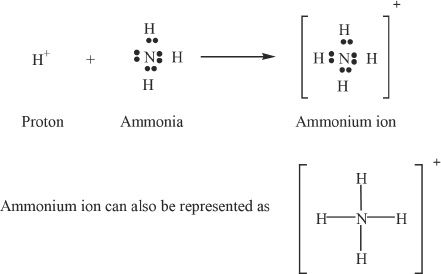
E. POLAR AND NONPOLAR COVALENT BONDS
When two atoms combine, just like in the formation of a hydrogen molecule, the atoms are one and the same and they have the same electronegativity. But, if the two atoms that are combined via covalent bond are different, then there is unequal sharing of electrons due to the electronegativity difference between those atoms. Such a bond is called a polar covalent bond. The former case (H2 molecule) is an example of a nonpolar covalent compound. In the HBr molecule, the bonding electrons will be more attracted to the more electronegative of the two atoms, namely bromine. So the bonding electrons are likely to spend more time closer to the more electronegative atom or group. Hence, this bond is polar.
![]()
The delta+ (δ+) indicates the partial positive charge of the hydrogen atom, and delta- (δ-) indicates the partial negative charge of the bromine atom. Small and equal charges being separated by a small distance constitute a dipole. The polarity is quantitatively represented in terms of dipole moment, which is the charge times the distance between the charges. Now that you know dipole moment, let’s learn how to represent this.
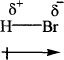
Here the net dipole moment is as indicated by the arrow.
When there are more than two dipoles, the net effect is the vector sum of all individual dipoles in the molecule. For example, in carbon dioxide (CO2) the net dipole moment is as shown below.
![]()
Since the dipoles are equal and opposite, the vectors of the dipoles cancel out.
F. THE LEWIS ELECTRON-DOT FORMULAS
Lewis Electron-Dot Formulas: Lewis electron-dot formulas are diagrammatic representations of the atoms involved and their valence electrons. The valence electrons are usually represented as dots around the elemental symbol. It is a two-dimensional way of representing the structural formula, showing the bonding electrons and the lone electrons that are in the valence shells.
Writing Lewis Formulas
The objective of this section is to become comfortable with writing Lewis structural formulas. We can only predict the Lewis structures of simple molecules. Other complex structures require complicated analysis and predictions based on experimental data.
1. First, determine the main structural make up of the molecule, such as guessing which atom will be the central atom of the molecule. The central atom of the molecule is usually the atom with the lowest electronegativity.
2. Next, determine the total number of valence (outermost) electrons.
3. Draw the basic skeletal structure of the molecule or ion.
4. Next, determine the distribution of those valence electrons so as to complete the octet of the atoms that are around or bonded to the central atom.
5. The remaining electrons are to be distributed in pairs around the central atom.
6. Sometimes you might find that the central atom is not reaching the octet level even at this point. The most likely reason is that there might be a need of a double bond or a triple bond.
Let’s go through an example. Before looking at the solution of the example shown below, determine the Lewis structure on your own.
Example 5-1
Write the Lewis dot formula of carbonate ion
(CO3)2—
Solution:
Since carbon is the least electronegative, it is the most likely atom to be in the center. With this information, we can draw the carbonate ion as indicated below.
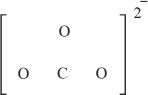
Next, calculate the number of valence electrons. Carbon has 4 electrons, whereas oxygen has 6 electrons each, and do not forget the net charge of -2, which accounts for two more electrons. This tallies to a total of 24 electrons. We can set up all these valence electrons around individual atoms in the structure drawn so far, as shown below:

Now that we have set all the valence electrons, we are done, right? No! Watch out for the carbon. The octet of carbon is not satisfied, and thus carbon will not be very stable with its valency at this point. Since there are no more electrons to spare, we have to move one of the pairs of electrons from an oxygen atom, which results in 1 double bond and 2 single bonds. The completed Lewis structure of the carbonate ion should look as follows:
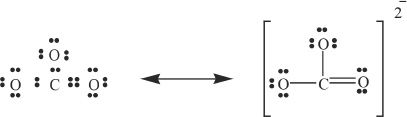
G. RESONANCE
Resonance is an important concept in chemistry. Though we represent definite Lewis structures of molecules, in actuality the electrons are not localized. They are shared and delocalized by atoms in such a way as to be in the most stable electron distribution. This is called resonance.

The actual form of these resonance structures is an average of all the possible resonance forms of the group or molecule.
H. FORMAL CHARGE
The concept of formal charge is based on the assumption that the electrons involved in the bonds are equally shared between the involved atoms. As you have already seen, this is not exactly the case. But for simplicity and from an analytical point of view, we assume that the electrons are equally distributed.
Formal charge of the atoms is the charge of those atoms in a formula, under the assumption that the electrons in the bonds are equally distributed between the atoms that contain the bond.
![]()
There are different ways to find formal charges. One way is mentioned here. You can either choose to do it this way, or whichever way you are comfortable with.
Example 5-2
Find the formal charges of all the atoms in thionyl chloride (SOCl2).
Solution:
The Lewis structure of thionyl chloride is written below:

Using the formal charge formula, it is just a matter of plugging in the numbers. Both oxygen and sulfur belong to Group VI. Chlorine belongs to Group VII. Sulfur has 3 bonds and 2 unshared electrons. Oxygen has 1 bond and 6 unshared electrons. Chlorine has 1 bond and 6 unshared electrons.
![]()
The formal charge of sulfur = 6 — 3 — 2 = +1
The formal charge of oxygen = 6 — 1 — 6 = —1
The formal charge of chlorine = 7 — 1 — 6 = 0
Notice that the net charge of the molecule is zero, as expected, since it is a molecule and not an ion.
Problem 5-1
Which of the following represents the formal charge of sulfur in sulfuric acid (H2SO4)?
A) —1
B) +1
C) —2
D) +2
Solution:
If you draw the structure, you’ll see that sulfur is the central atom, and has 2 single bonds to the 2 oxygen atoms, and 2 single bonds to the OH groups. The answer is +2.
I. BOND LENGTH AND BOND ENERGY
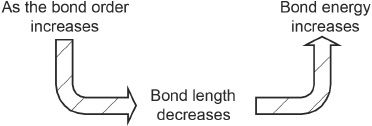
Bond length is the distance between the nuclei of the atoms that are bonded together. Bond energy is the amount of energy required to pull the atoms involved in the bond away from each other. Bond order is the number of bonds (covalent) that exist between the two bonded atoms. The bond length and bond order are inversely proportional. Hence, as the bond order increases, the bond length decreases and the bond energy increases.
Problem 5-2
Which of the following will have the highest bond energy?
A) A carbon-carbon single bond
B) A carbon-carbon double bond
C) A carbon-carbon triple bond
D) A carbon-hydrogen bond
Solution:
The bond order of a triple bond is 3. Carbon-carbon single bond and carbon-hydrogen bond have bond orders of 1. This eliminates choices A and D. The bond order of carbon-carbon double bond is 2. The one with the highest bond order will have the highest bond energy. So the answer is choice C.
J. MOLECULAR GEOMETRY
In this section, we will study the valence shell electron pair repulsion theory and the basis for predicting the shapes of some structures.
Valence Shell Electron Pair Repulsion Theory
Valence shell electron pair repulsion theory (VSEPR) can be used to predict the shapes of molecules. According to this theory, the geometry of a molecule is such that the valence-electron pairs of the central atom are kept farthest apart to minimize the electron repulsions. Again, you have to view molecules in terms of Lewis structure so that the shape of the molecules can be predicted with the VSEPR theory.
Molecular geometry of a molecule is the directional orientation of the bonded pairs around the central atom, excluding the unshared electron pairs.
Let’s look at the practical significance of this theory through some examples.
Example 5-3
Predict the molecular geometry of the CO2 molecule.
Solution:
Carbon, obviously the central atom, has four electrons in its valence shell. In order to complete the octet, carbon requires four more electrons or two pairs of electrons. Oxygen atom has six electrons in its valence shell. In order to complete its octet, each oxygen atom requires two electrons or one pair of electrons. The Lewis structure of CO2 should look like the figure shown below. Note that the valence electrons of the carbon atom are denoted by asterisks (*) and that of the oxygen are denoted by dots.
![]()
The molecule keeps this linear shape, so that the electrons are placed far apart as predicted by the VSEPR theory. Also notice that the carbon-oxygen bonds are double bonds. Hence the CO2 molecule is linear in shape.
Example 5-4
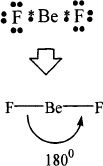
Predict the shape of the BeF2 molecule.
Solution:
Beryllium has two electrons in its outer most shell. In this case, there is an exception to the octet rule, because in the formation of the molecule, only two pairs (four electrons) are present in the valence shell of beryllium. Each fluorine atom requires one electron to attain the complete octet. Take a look at the structure shown below. Again for clarity, beryllium electrons are denoted by asterisks and fluorine electrons are denoted by dots.
Example 5-5
Predict the shape of the water (H2O) molecule.
Solution:
When dealing with bond formation with the possibility of existence of lone pairs, we have to take that into consideration. You will see that in this example. The oxygen atom has six valence electrons, and the hydrogen atom has one valence electron. So in the structural formation, there are two lone pairs in the central oxygen atom. How do you think this will change the shape of the molecule? Well, because of the lone pairs, the molecule will be bent, rather than linear. The structure is shown below.

Problem 5-3
Predict the geometry of the following molecules.
A) BeCl2
B) NH3
C) SiCl4
D) BF3
E) CH4
Answers:
A) linear
B) trigonal pyramidal
C) tetrahedral
D) trigonal planar
E) tetrahedral
Table 5-1
SOME COMMON GEOMETRIES AND THEIR CHARACTERISTICS
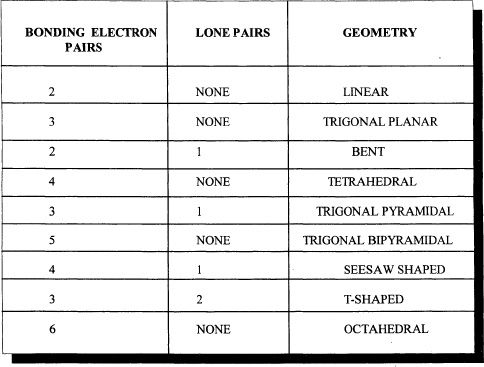
CHAPTER 5 PRACTICE QUESTIONS
1. Which of the following best represents the Lewis structure of SO3—2?
2. Which of the following has a net dipole moment of zero?
A. H2O
B. HCl
C. CO
D. CH4
3. The bond between nitrogen and oxygen in NO2 is most likely:
A. an ionic bond.
B. a covalent bond.
C. a hydrogen bond.
D. a dipole-dipole interaction.
4. Ammonia (NH3) can bond with boron trifluoride (BF3). For the bond formation, the electrons are most likely supplied by:
A. nitrogen.
B. boron.
C. both boron and nitrogen because the bond formed is covalent.
D. hydrogen.
5. Which of the following has the highest dipole moment?
A. HF
B. CCl4
C. HBr
D. CO2
6. The molecular geometry of BCl3 is:
A. linear.
B. trigonal planar.
C. tetrahedral.
D. trigonal bipyramidal.
7. CCl4 molecule is:
A. trigonal planar.
B. linear.
C. octahedral.
D. tetrahedral.
8. Which of the following best describes the geometry of nitrate (NO3—)?
A. Trigonal planar
B. Trigonal bipyramidal
C. Tetrahedral
D. Angular
9. What is the molecular geometry of SF6?
A. Hexagonal
B. Tetrahedral
C. Octahedral
D. Trigonal planar
10. CH4 and SiF4 are examples of tetrahedral geometry. Which of the following are true regarding these two molecules?
I. The carbon-hydrogen bonds are dipoles
II. The silicon-fluorine bonds are dipoles
III. Methane is nonpolar
IV. SiF4 has zero dipole moment
A. I & II only
B. II only
C. II & III only
D. I, II, III & IV
11. Which of the following types of bonds has the least electronegativity difference between the bonding atoms?
A. Ionic bond
B. Polar covalent bond
C. Nonpolar covalent bond
D. Cannot be predicted without the actual electronegativity data.
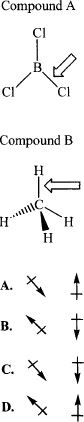
12. Which of the following choices represents the vectors of bond dipoles of the indicated (indicated by the arrows) bonds of the compounds A & B, respectively?
13. Choose the answer which best denotes the electronic orientation (electronic geometry) of the water molecule.
A. Angular
B. Bent
C. Trigonal planar
D. Tetrahedral