The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Gases
A. INTRODUCTION
In this chapter, we will review the essential aspects of gases, and the terms that are commonly associated with them. This chapter will also discuss the various laws such as Charles’ law, Boyle’s law, and ideal gas law. Finally, the kinetic law of gases will be explored.
B. GASES — AN OVERVIEW
Gases are unique when compared to liquids and solids. Gases can be compressed into smaller volumes, and they can be mixed extensively. They have relatively very low densities. Ideally, a gas exerts pressure on all sides of the container it occupies in a uniform manner. These are some of the properties of gases. Let’s explore these ideas in detail.
Standard Temperature and Pressure
It is very important to understand the general aspects of gas pressure, its measurements, and calibrations. Pressure is force exerted over unit area. The unit of pressure is pascal (Pa) which is equivalent to kg/(m.s2). In chemistry, we usually use a mercury-based barometer to measure the pressure. The unit is millimeter of mercury (mmHg). This is the same as the unit torr. Another unit commonly used to denote pressure is atmosphere (atm). You should be able to convert these units back and forth as required.
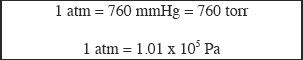
Gases can be compressed or expanded by adjusting the temperature and other conditions that prevail. In order to standardize the quantities of gases measured and conveyed, arbitrary reference conditions called standard temperature and pressure (STP) have been chosen and internationally accepted. The specific values of temperature and pressure at STP are 0° C (273 K) and 1 atm (760 mmHg).

You should know what STP is, because in many questions and passages, the actual temperature and pressure situations will not be explicit. Rather, they will say, for example, that the reaction was undertaken at standard temperature and pressure. This means, you have to automatically know that they are talking about the temperatures corresponding to STP reference conditions, which you already know.
Molar Volume
Ideally, one mole of gas at STP occupies a volume of 22.4 L. This is known as molar volume. We should bear in mind the fact that this is under standard conditions. Do the example below on your own, before you look at the solution.
Example 6-1
Calculate the number of moles of oxygen at STP present in a volume of 78.75 L.
Solution:
One mole of gas occupies a volume of 22.4 L. To get the number of moles, you have to divide 78.75 L by 22.4 L/mol.
![]()
C. GAS LAWS
Gases behave ideally under reasonably high temperatures and low pressures. The gas laws are helpful in quantitatively relating pressure, volume, temperature, and molar units.
Boyle’s Law
According to Boyle’s law, the volume of a fixed amount of gas is inversely proportional to the pressure, provided that the temperature is kept constant. A simple and good example with biological significance is the way we take air into our lungs. The way we breathe can be summarized as follows. As the respiratory centers signal, during inspiration the diaphragm contracts resulting in an increase in the thoracic volume, which in turn translates into an increase in the lung volume. This increase in lung volume results in a decrease in pressure. This decrease in pressure inside the lung results in the rushing of air into the lung from the outside — inspiration. The exact opposite conditions result in expiration. The point is that at constant temperature, the volume of a gaseous sample is inversely proportional to its pressure.
![]()
This can be represented mathematically as shown below:
![]()
Example 6-2
200 ml of a gas is present in a cylinder at a pressure of 760 torr. If the gas is compressed by using a piston to a pressure of 950 torr, calculate the final volume occupied by the gas. (Assume the temperature to be constant)
Solution:
This problem specifically tests your knowledge of Boyle’s law. We know that PV is a constant, provided that the temperature is kept constant. Since the temperature is constant, we can readily apply Boyle’s law. We can equate the initial and final stages of the system. P1 and V1 represent the initial pressure and volume respectively. P2 and V2 represent, the final pressure and volume respectively.
![]()
Now it is just a matter of solving for V2 (final volume).
![]()
Charles’ Law
In 1787, Jacques Charles showed that gas expands to occupy a larger volume as the temperature increases. Volume can be plotted against the temperature as shown below:
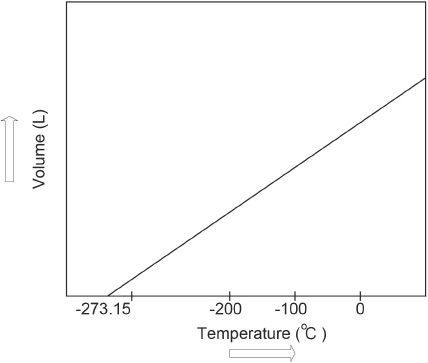
Figure 6-1
When the temperature is increased, the volume increases. Charles found that the volume of a gas is directly proportional to its absolute temperature, provided that the pressure is kept constant. So the volume and temperature have a linear relationship as represented by the graph. Charles’s law can be mathematically expressed as follows:

The volume-temperature graph shows that at zero volume, the corresponding temperature value is —273.15° C. This means that at —273.15° C, the volume occupied by the gas is zero. This temperature is unique, and scientists so far have not been able to devise a way to lower the temperature to —273.15° C.
Combined Gas Law
The gas laws (Charles’ and Boyle’s law) can be combined to form the combined gas law. The resulting law can be represented mathematically as shown below:
![]()
This relationship can be used to do a variety of calculations involving gases, since it relates pressure, volume, and temperature.
The Ideal Gas Law
This is an extension of the combined gas law. In the combined gas law, we saw the relationship between pressure, volume, and temperature. The ideal gas law can be expressed mathematically as follows:

Here, R (molar gas constant) has values 0.082 L.atm/(K.mol), or 8.31 J/(K.mol), and of course the difference in values is due to the fact that the gas constant is expressed here in two different units.
D. KINETIC THEORY OF GASES
The kinetic theory of gases can be explored in terms of the following assumptions. The theory tries to answer the questions regarding the various properties of gases. It also gives the relationship between the kinetic energy of the molecules and the absolute temperature. The important points in kinetic theory of gases are listed below:
1. A gas consists of small particles (sizes are considered relatively negligible) known as molecules, which are separated widely apart and thus the gas container is mostly empty.
2. The molecules are in a state of continuous random motion, and colliding against each other and also against the sides of the container, and furthermore the collisions are elastic.
3. These collisions result in the pressure of the gas.
4. The molecules are expected to travel in straight lines at different speeds in all directions.
5. The forces (attractive or repulsive) between the molecules are negligible.
6. The average kinetic energy of the molecules is proportional to the temperature.
Ideal gas molecules travel randomly in straight lines at different speeds.
Some Aspects Related to Kinetic Theory
The main aspect of kinetic theory is that the molecules in a gas are in a state of continuous random motion. The speed of molecules depends on the temperature, and can have a range of values. Maxwell’s distribution curve is perfect to analyze this fact. In the Maxwell’s distribution curve, the relative number of molecules are plotted against the molecular speed on the x-axis. Take a look at the curve in Figure 6-2.
Note that the speed corresponding to the maximum number of molecules is called the most probable speed. This is always smaller than the average speed, which is in turn smaller than the root-mean-square speed (rms speed).
The formula for root-mean-square speed is given below:
![]()
Here, R is the gas constant, T is the absolute temperature, and M is the molar mass of the gas.
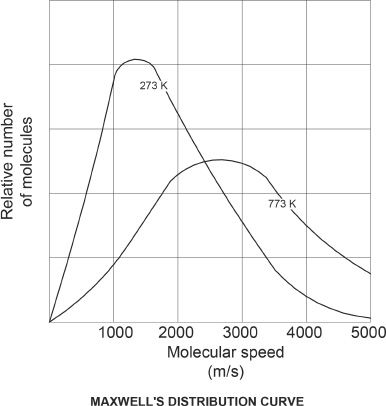
Figure 6-2
E. PARTIAL PRESSURE AND MOLE FRACTION
Let’s consider a container which has different gases in it. According to the Dalton’s law of partial pressures, the partial pressures of the gases present in a gaseous mixture can be added to get the net total pressure of the mixture. But, what is partial pressure? Partial pressure is the pressure exerted by a particular gas in a mixture of gases. Now that you know what partial pressure is, take a look at the boxed information which summarizes the whole idea of Dalton’s law of partial pressures.
Dalton’s Law of Partial Pressures
Ptotal = PA + PB + PC + PD + … (at constant volume & temperature)
Ptotal_ — Total pressure
PA, PB, PC, PD, … represent the partial pressures of gases A, B, C, D, and so on.
Another concept you have to understand is mole fraction. The mole fraction of a gas is the fraction or ratio of moles of that particular gas against the total number of moles of gases present in the mixture. Mole fraction is defined as follows:

Example 6-3
A 1 liter flask contains 0.4 mol of helium and 1.2 moles of hydrogen gas. Find the mole fractions and partial pressures of both gases, if the total pressure of the mixture is 790 mmHg.
Solution:
The total number of moles of gases present in the container is 0.4 + 1.2 = 1.6 moles
The mole fraction of helium = 0.4/1.6 = 0.25
The mole fraction of hydrogen = 1.2/1.6 = 0.75
Notice that the sum of the mole fractions is always one. If it is not, you probably made an error somewhere in your calculation. Here,
0.25 + 0.75 = 1.0
Next, we have to find the partial pressures of the gases. We know that the partial pressures of the gases should add up to get the total pressure of the gases. Now that we know the total pressure and the mole fractions, we can calculate the partial pressures of helium and hydrogen.
Partial pressure of gas A = mole fraction of gas A x total pressure
Partial pressure of helium = 0.25 x 790 mmHg = 197.5 mmHg
Partial pressure of hydrogen = 0.75 x 790 mmHg = 592.5 mmHg
F. GRAHAM’S LAW
Diffusion of gases can be described as the process by which a gas spreads to occupy the available and accessible space, thereby creating a uniform pressure throughout the space the gas occupies. A gas having a higher partial pressure will travel or diffuse toward regions of gases having a lower partial pressure, until an equilibrium is reached. To analyze the diffusion of gases, it is much simpler to think in terms of effusion. The difference between diffusion and effusion is that diffusion is the movement of gas through the entire volume of the container, whereas effusion is the movement of gas through a tiny hole of the container.
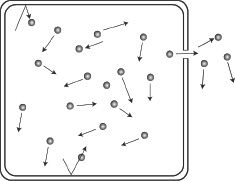
Figure 6-3
The figure depicts the effusion of gas molecules through a small hole in a container.
According to Graham’s law of effusion, the effusion rate of a gas is inversely related to the square root of the molecular weight of the gas. This is true provided that we have the same pressure and temperature conditions. Graham’s law can be expressed as shown below:

Graham’s law can also be applied to diffusion.
Example 6-4
Calculate the ratio of effusion of the gases oxygen and hydrogen. Assume that the two gases are in the same container having a tiny hole in it.
Solution:
First, we have to consider the fact that according to Graham’s law, the rate of effusion is inversely proportional to the square of molecular weight of the gas, which is effused. This can be represented as follows. Keep in mind that both gases are diatomic.
![]()
So the ratio of the rates of effusion of hydrogen and oxygen is 4 : 1.
We should think about the implications of this ratio. This means that the hydrogen molecules will effuse four times faster than the oxygen molecules.
CHAPTER 6 PRACTICE QUESTIONS
1. Consider the reaction between nitric oxide and oxygen to form nitrogen dioxide. If 4 liters of nitrogen dioxide were formed, how many liters of nitric oxide and oxygen must have reacted?
A. 2 L of NO & 1 L of O2
B. 2 L of NO & 2 L of O2
C. 4 L of NO & 2 L of O2
D. 2 L of NO & 4 L of O2
2. At a particular temperature and pressure, 1 mol of gas M occupies 45 liters. If the density of gas M at the same temperature and pressure is 1.8 g/L, the molecular weight of gas M is:
A. 25 g/mol.
B. 81 g/mol.
C. 40.5 g/mol.
D. 4 g/mol.
3. A certain gas behaves ideally at STP. If the density of the gas is 1.4 g/L, what is the most likely identity of this gas? (The gas is diatomic.)
A. SO2
B. N2
C. O2
D. F2
4. A hypothetical reaction R occurs only at elevated pressures. It was noted that the optimum pressure required for the reaction was 1330 mmHg. This value is roughly equal to: (1 atm = 101.3 kPa)
A. 1.3 x 10—2 Pa.
B. 1.8 x 105 Pa.
C. 1.35 x 108 Pa.
D. 1.8 x 102 Pa.
5. —270 C is the same as:
A. 300 K.
B. 246 K.
C. 270 K.
D. 127 K.
6. The volume occupied by 11.5 g of carbon dioxide at STP is approximately equal to:
A. 5.9 L.
B. 22.5 L.
C. 86 L.
D. 259 L.
Questions 7-13 are based on the following passage.
Passage 1
The behavior of gases can be predicted to a large extent on the basis of various laws. Gases are expressed mainly in terms of pressure, volume, and temperature. Many gases obey the ideal gas laws and those gases are called ideal gases. If we are not doing precision experiments, we can normally ignore the slight deviations that occur under normal conditions. Nevertheless, we cannot completely ignore the deviation factors.
According to Boyle’s law, the pressure is inversely proportional to the volume at a constant temperature. Charles’ law states that the volume is directly proportional to the temperature at a constant pressure. Combining these laws and Avogadro’s law gives the combined gas law.
PV = nRT
(The ideal gas law)
Not all gases behave ideally. There are often deviations from the ideal behavior. At low temperatures, gases often behave differently apart from what is described by kinetic-molecular theory of gases. Considering these correction factors, the modified gas equation is:
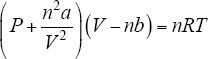
The correction constants or Vander Waal constants, a and b, of gases are experimentally found. The Van der Waal constants of some gases are given in Table 1.
Table 1
Gas |
a (L2.atm/mol2) |
b (L/mol) |
Carbon monoxide |
1.470 |
0.039 |
Hydrogen |
0.245 |
0.026 |
Chlorine |
6.340 |
0.054 |
Fluorine |
1.170 |
0.029 |
Hydrogen sulfide |
4.540 |
0.043 |
Nitrogen |
1.380 |
0.039 |
Carbon dioxide |
3.660 |
0.043 |
Argon |
1.360 |
0.032 |
Propane |
9.390 |
0.091 |
7. Under which of the following conditions will gases behave most ideally?
A. Low temperature and high pressure
B. High temperature and low pressure
C. Low temperature and low pressure
D. Gas behavior does not depend on temperature and pressure if the gas is an ideal gas, and such a gas will behave ideally regardless of the conditions
8. What is the unit of universal gas constant R?
A. ![]()
B. ![]()
C. ![]()
D. ![]()
9. A group of general chemistry students were assigned to conduct gas experiments. The vessel that contained the gases had a small hole in it. The gases used in the experiments were SO2 and O2. What is the rate of leakage of SO2 to O2 through the hole?
A. 0.7
B. 4
C. 2
D. 1.4
10. If the Vander Waal constant a is found to be zero for gas A, what are true regarding this gas?
I. Gas A does not behave ideally.
II. Gas A behaves ideally.
III. Gas A is Xenon.
A. I only
B. II only
C. II & III only
D. III only
11. What is the most likely Van der Waal constant correction factor of bromine?
A. 0.97 L2.atm/mol2
B. 2.34 L2.atm/mol2
C. 5.32 L2.atm/mol2
D. 9.80 L2.atm/mol2
12. Equal volumes of gases will contain the same number of molecules at the same temperature and pressure. This prediction is directly based on:
A. Charles law.
B. Boyle’s law.
C. kinetic-molecular theory.
D. Avogadro’s law.
13. Based on kinetic-molecular theory, which of the following are true?
I. At a given temperature, all gases have the same average kinetic energy.
II. At a given temperature, different gases have different average velocities.
III. The average kinetic energy is proportional to the absolute temperature.
A. I only
B. II only
C. I & III only
D. I, II & III