The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Chemistry of Solutions
A. INTRODUCTION
When we think of solutions, we normally think of a solution formed by dissolving a solid (e.g., sugar) in a liquid (e.g., water). In reality, any combination of the three states can be considered a solution. In fact, air is a solution which is a mixture of various gases. Carbonated water (soda) is a mixture of a gas (CO2) dissolved in a liquid (H2O). Even alloys such as gold-silver alloys are solutions containing two solids. A true solution is a solution which has only one solvent with one or more solutes. In this chapter, we will be limiting our discussion mostly to water based solutions because of their versatility and importance to life sciences. We will discuss various aspects such as solubility of compounds, and precipitation reactions.
B. THE CONCEPT OF SOLUBILITY
Solubility of a substance is defined as the amount of the substance that will dissolve in a particular solvent. Let’s consider an example in which sucrose is added to water. When we add sucrose and stir, it dissolves in the water. Let’s say we keep on adding more and more sucrose to the solution. Since more and more sucrose is dissolving, the solution is not saturated or it is called an unsaturated solution.
It reaches a point where additional amount of sucrose will not dissolve in that solution. At that point the solution is said to be saturated. We have yet another category in this tradition of categorizing solutions. In some cases, certain solutes become more soluble if the solution is heated. Such solutions are called supersaturated solutions. If we carefully and slowly cool down a supersaturated solution without disturbing its contents, normally we will still have a supersaturated solution. At this stage, even the slightest addition of the solute will result in crystallization.
The Reasons for Solubility
One basic rule that you have to keep in mind is “like dissolves like.” This means that substances which have similar polarity dissolve one another. We know that water is a polar substance. Since oil is nonpolar, water and oil cannot mix. Solubility helps in maintaining the lowest energy possible when the solute and the solvent are mixed together.
Ionic Solutions
Ionic compounds have very peculiar solubility trends. Some are highly soluble, whereas some others have very little solubility. The solubility of ionic compounds can be explained in terms of the interactions between the ions and the water molecules. Let’s take sodium chloride as an example. Sodium chloride has a solubility of 360 g per liter or 36 g per 100 ml at room temperature.
Since water is a polar molecule, when we add sodium chloride to water, the water molecules will orient themselves according to the surrounding ions. Here we have sodium (Na+) and chloride (Cl—) ions. So the slightly negative pole (the negative pole is due to the electronegativity of the oxygen atom) of the water molecules will align toward the sodium ions, and the other pole will orient toward the chloride ions. This attraction is otherwise called hydration. Besides hydration, there is another force called lattice energy of the crystal lattice. The greater the lattice energy of an ionic solid is, the lesser its solubility.
C. MOLARITY
When we are working with solutions, it is better to have a concentration measure in terms of the volume. Molarity is defined as the number of moles of the solute per liter of solution.
![]()
D. MOLALITY
The molality of a solution is defined as the number of moles of the solute per kilogram of solvent.
![]()
Molarity is usually denoted by the letter M, and molality is denoted by the letter m.
Example 8-1
Calculate the molarity of a solution that contains 18.65 g of KCl in 3 liters of solution.
Solution:
We have 18.65 grams of KCl, and the total volume of the solution is 3 liters. First, find the number of moles of KCl present. The number of moles of KCl is 0.25 mol, which can be calculated from the formula weight and the grams of solute.
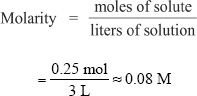
E. NORMALITY
The normality (N) of a solution is the number of equivalents of solute per liter of solution. The equivalent is usually defined in terms of a chemical reaction. For acid-base reactions, an equivalent is the amount of substance that will react or form 1 mole of hydrogen (H+) or hydroxide (OH—) ions. For redox (oxidation-reduction) reactions, an equivalent is the amount of substance that will react or form 1 mole of electrons.
![]()
Normality is a multiple of molarity. The following equation relates normality and molarity.
N = n M
For acids, the number of H+ available from a formula unit of the acid gives the number of equivalents (n). For example, 1 M H2SO4 solution is a 2 N solution, because each molecule of H2SO4can give two H+. For bases, the number of OH— available from a formula unit of the base gives the number of equivalents. For example, a 2 M Ca(OH)2 solution is a 4 N solution.
F. THE SOLUBILITY PRODUCT CONSTANT
Let’s say we are adding silver chloride (AgCl) into a liter of water. We can express the equilibrium as follows:
![]()
The solubility product (Ksp) is defined as the equilibrium constant for the solubility equilibrium of ionic compounds. The solubility product of AgCl is expressed as:
![]()
The solubility product constant is equal to the product of the concentrations of the ions in a saturated solution, each concentration raised to a power equal to its coefficient (number of moles of individual ions formed per mole of the compound) Take a look at the solubility product equation of calcium fluoride (CaF2).
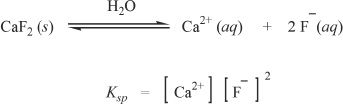
It is imperative that you understand these ideas involving the solubility product constant. Let’s look at another example. One mole of aluminum hydroxide [Al(OH)3] gives one mole of aluminum ions and three moles of hydroxide ions in solution.
![]()
From the balanced equation, we can see that the coefficient of Al3+ is 1 and that of OH— is 3. So, the solubility constant (Ksp) expression for Al(OH)3 is given by:
Ksp = [Al3+] [OH—]3
The solubility product depends on the temperature, and at a given temperature it is constant for a particular ionic compound. Molar solubility is defined as the number of moles of solute dissolved in one liter of its saturated solution. Using the molar solubility of a compound, the solubility product of that compound can be determined or vice versa.
Example 8-2
Calculate the molar solubility of silver chloride (AgCl). The solubility product (Ksp) of AgCl is 1.8 x 10—10.
Solution:
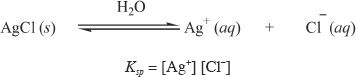
Let x be the molar solubility of AgCl. So we can write:
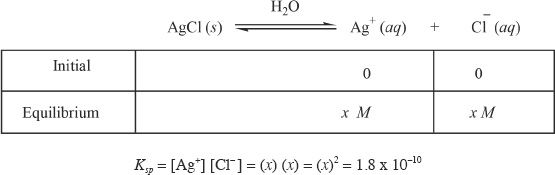
So the molar solubility of AgCl, ![]()
Example 8-3
If ’x’ represents the molar solubility of Al(OH)3, find the expression for the solubility product of Al(OH)3 in terms of x. What is the molar solubility of Al(OH)3, if the solubility product constant is 2 x 10—33?
Solution:
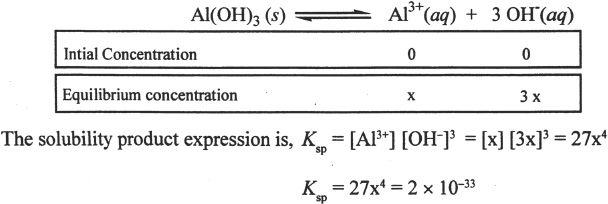
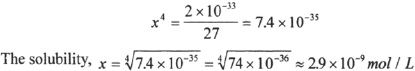
You can approximate numbers to find the correct answer among the choices in the MCAT. In this example, to the 4th root of 7.4 x 10—35, first we can rewrite this as 74 x 10—36. The 4th root of 81 is 3. Since 74 is less than 81, we can approximate the 4th root of 74 close to 2.8 or 2.9. If you had trouble realizing that, think of the 4th root of 16 which is 2. Again, the best approximation for the 4th root of 74 is a number below and close to 3. The 4th root of 10—36 is 10—9. Such approximation-techniques can save time during the test.
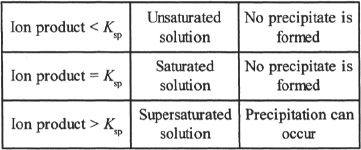
Ion Product
Ion product is the product of the concentrations of the ions from the compound (solute) in a solution, each concentration raised to a power equal to its coefficient in the balanced equation. In other words, the expression for the ion product is the same as that of Ksp. In a saturated solution, the ion product is equal to Ksp. If the ion product is greater than Ksp, the solution is supersaturated and can undergo precipitation. If the ion product is less than Ksp, the solution is unsaturated. Thus, by comparing the ion product of a compound against its Ksp, we can predict whether or not precipitation is likely to occur.
G. COMMON-ION EFFECT
When a salt is added to a solution containing either the same cation or anion, there will be changes in the solubility because of what is commonly known as common-ion effect. This is so because the solubility of the salt added is affected by the common ions which are already present in the solution. Consider a solution of sodium fluoride (NaF). We are adding magnesium fluoride (MgF2) to this solution. Notice the fact that there is a common ion in sodium fluoride and magnesium fluoride, namely fluoride. You will see that the solubility of magnesium fluoride will be less than expected. To be more precise, the solubility of magnesium fluoride will be less than that of its solubility in pure water. Why is it so? Well the answer is simple; common-ion effect. The phenomenon can be best explained in terms of Le Chatelier’s principle. Consider the dissociation of magnesium and sodium fluorides.
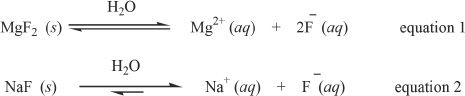
Magnesium fluoride is not a very soluble salt. It is a mildly soluble salt, whereas sodium fluoride is a highly soluble salt. Because of the higher solubility of sodium fluoride, there will be a lot of fluoride ions in the solution. This increased fluoride ion concentration in the solution will drive the equilibrium of Equation 1 to the left. Thus the solubility of magnesium fluoride will be highly diminished because of the fluoride ions already present in the solution.
Table 8-1 Some general trends regarding solubilities (in water) of common ionic compounds
SOLUBLE IONIC COMPOUNDS |
All salts of alkali metals (e.g., sodium, potassium), and ammonium ion are soluble. |
All nitrates, acetates, chlorates, and perchlorates are soluble. |
All halides (chlorides, bromides, and iodides) are soluble. Exceptions include lead(II), mercury (I), and silver halides. |
All sulfates are soluble. Exceptions include calcium, strontium, barium, and lead(II) sulfates. |
INSOLUBLE IONIC COMPOUNDS |
All carbonates and phosphates are insoluble except those of ammonium ion and alkali metals. |
All hydroxides are insoluble except those of calcium, strontium, barium, and alkali metals. |
All sulfides are insoluble except those of alkali metals and ammonium ion. |
H. ELECTROLYTES
Pure distilled water is not a good conductor of electricity. If KCI is added to it, the resulting aqueous KCI solution becomes a good conductor of electricity. Such a substance that dissolves in water to give a solution that can conduct electricity is called an electrolyte. The free ions that are present in an electrolytic solution enable the flow of electric current. Most ionic substances are good electrolytes because they dissolve in water ions. A substance that exists almost completely as ions in a solution is called a strong electrolyte. Hydrochloric acid is a strong electrolyte because when HCI is dissolved in water, it completely exists as H3O+ and Cl— ions by reacting with water (H2O).
![]()
Some substances (solutes) form aqueous solutions that weakly conduct electricity. Such substances are called weak electrolytes. This weak conduction of electricity is mainly because weak electrolytes only dissociate partially in solution. In other words, only a small fraction of the solute exists as ions, resulting in a solution that conducts electricity very weakly. Acetic acid (CH3COOH) and ammonia (NH3) are examples of weak electrolytes.
A substance that can dissolve in water but results in a poorly conducting solution is called nonelectrolyte. Glucose (C6H12O6) is a nonelectrolyte. A nonelectrolyte is not charged in solution because it dissolves as molecules in water rather than ions, and thus cannot conduct electricity.
Table 8-2
CHAPTER 8 PRACTICE QUESTIONS
1. Equivalents of solute per liter of solution is:
A. molality.
B. molarity.
C. normality.
D. none of the above.
2. The concentration of a given solution of glucose (C6H12O6) is 270 g per liter of solution. What is the molarity of this solution?
A. 1.5 M
B. 0.67 M
C. 4.86 M
D. 270 M
3. You are presented with a 2.5 M solution of hydrochloric acid. If the total volume of the solution is 1.25 L, how many grams of HCl is present in this solution?
A. 36.5 g
B. 91.25 g
C. 111 g
D. 114 g
4. You are asked to prepare 30 ml of a 2 M solution of HNO3 from a stock solution of 10 M solution of HNO3. How much of the stock solution is required to make this 30 ml of 2 M HNO3 solution?
A. 3 ml
B. 6 ml
C. 10 ml
D. 30 ml
5. How many grams of NaCl are required to prepare 250 ml of 0.35 M NaCl solution?
A. 0.0875 g
B. 20.5 g
C. 5.1 g
D. 87.5 g
Questions 6-10 are based on the following passage.
Passage 1
The concentration of solutions are generally expressed in terms of molarity, molality, normality, and weight percent. The formation of the solutions itself has many ramifications. The solubility of solutes differ considerably from one other. Some of the factors that can influence the solubility include temperature and pressure. Solubility depends on other factors as well. Given below in Figure-1 is a graph which depicts solubility differences of some solutes.
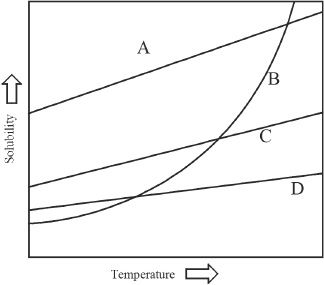
Figure-1
Quite often, the freezing and boiling point changes that are brought about by the dissolved solutes can be predicted reasonably. But this is not always the case.
The predictions and calculations are done for freezing point depression (ΔTf) based on the following formula:
ΔTf = Kf m,
where m is the molality and Kf is the freezing point depression constant. (Kf = 1.86 °C/m)
For boiling point elevation (ΔTb), the calculations are based on the following formula:
ΔTb = Kb m,
where m is the molality and Kb is the boiling point elevation constant. (Kb = 0.512 °C/m)
6. The graph in the passage shows the solubilities of some compounds (salts). Which of the following is most likely true regarding the Compounds A, B, C and D?
A. The solubility process of Compound B is exothermic, and those of Compounds A, C and D are endothermic.
B. The solubility process of Compound B is endothermic, and those of Compounds A, C and D are exothermic.
C. The solubility processes of Compounds A, B, C and D are exothermic.
D. The solubility processes of Compounds A, B, C and D are endothermic.
7. A solution was made by using 315 g of glucose in 750 g of water. What is the boiling point of this solution?
A. 98.80 C
B. 99.80 C
C. 100.240 C
D. 101.20 C
8. Two experiments were conducted for analyzing the solubility properties and their effects on colligative properties. In Experiment I, a 1.0 m solution of KBr was analyzed and in Experiment II, a 2.3 m solution of KBr was analyzed. The experimental values of freezing and boiling points were slightly different from the expected values based on the theoretical calculations discussed in the passage. Which of the following is most likely correct regarding the two experiments?
A. The experimental values of the solution in Experiment I was more different from the theoretical calculations than the solution in Experiment II.
B. The experimental values of the solution in Experiment II was more different from the theoretical calculations than the solution in Experiment I.
C. Both experiments have the same extent of differences from the theoretical predictions.
D. The discrepancy noted in the experiments is absolutely a result of instrumental error, because the differences in Experiments I & II cannot change colligative properties.
9. If the amount of glucose used in Question 7 is doubled, while the same 750 g of water was used, what must have happened to the boiling point of the solution?
A. It doubled.
B. It quadrupled.
C. It decreased slightly.
D. It increased slightly.
10. Which of the following statements is true regarding solutions?
A. As the concentration of a solution increases, the vapor pressure increases.
B. As the concentration of a solution decreases, the vapor pressure decreases.
C. As the concentration of a solution increases, the vapor pressure decreases.
D. None of the above