MCAT General Chemistry Review - Steven A. Leduc 2015
MCAT Math for General Chemistry
Logarithms
15.1 THE DEFINITION OF A LOGARITHM
A logarithm (or just log, for short) is an exponent.
For example, in the equation 23 = 8, 3 is the exponent, so 3 is the logarithm. More precisely, since 3 is the exponent that gives 8 when the base is 2,
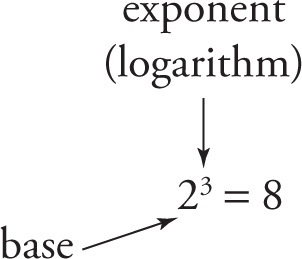
we say that the base-2 log of 8 is 3, symbolized by the equation log2 8 = 3.
Here’s another example: Since 102 = 100, the base-10 log of 100 is 2; that is, log10 100 = 2. The logarithm of a number to a given base is the exponent the base needs to be raised to give the number. What’s the log, base 3, of 81? It’s the exponent we’d have to raise 3 to in order to give 81. Since 34 = 81, the base-3 log of 81 is 4, which we write as log3 81 = 4.
The exponent equation 23 = 8 is equivalent to the log equation log2 8 = 3; the exponent equation 102 = 100 is equivalent to the log equation log10 100 = 2; and the exponent equation 34 = 81 is equivalent to the log equation log3 81 = 4. For every exponent equation, bx = y, there’s a corresponding log equation: logb y = x, and vice versa. To help make the conversion, use the following mnemonic, called the two arrows method:
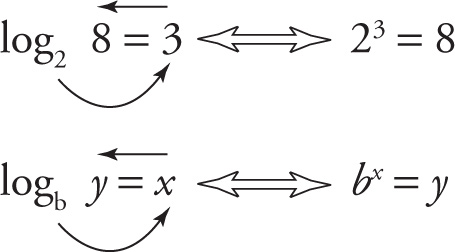
You should read the log equations with the two arrows like this:
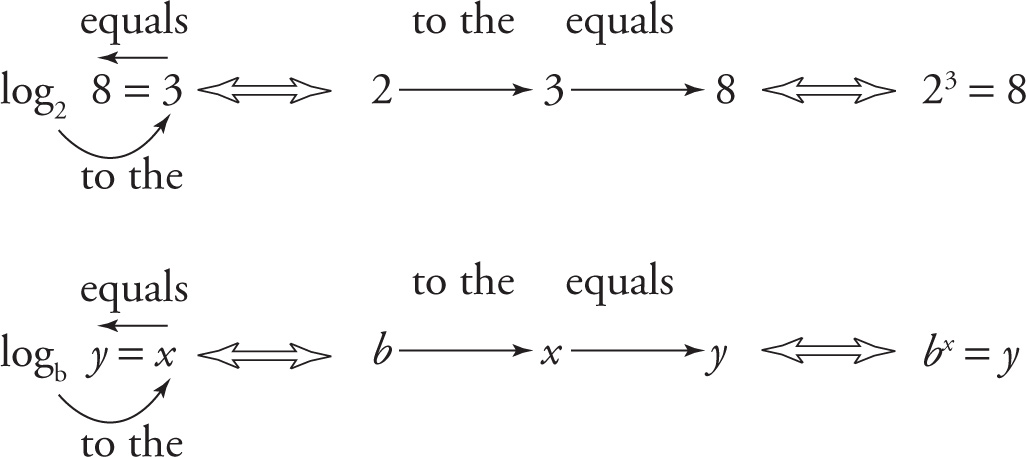
Always remember: The log is the exponent.
15.2 LAWS OF LOGARITHMS
There are only a few rules for dealing with logs that you’ll need to know, and they follow directly from the rules for exponents (given earlier, in Chapter 1). After all, logs are exponents.
In stating these rules, we will assume that in an equation like logb y = x, the base b is a positive number that’s different from 1, and that y is positive. (Why these restrictions? Well, if b is negative, then not every number has a log. For example, log—3 9 is 2, but what is log—3 27? If b were 0, then only 0 would have a log; and if b were 1, then every number x could equal log1 y if y =1, and no number x could equal log1 y if y ≠ 1. And why must y be positive? Because if b is a positive number, then bx [which is y] is always positive, no matter what real value we use for x. Therefore, only positive numbers have logs.)
|
Laws of Logarithms |
|
Law 1 |
The log of a product is the sum of the logs: |
Law 2 |
The log of a quotient is the difference of the logs: |
Law 3 |
The log of (a number to a power) is that power times the log of the number: |
We could also add to this list that the log of 1 is 0, but this fact just follows from the definition of a log: Since b0 = 1 for any allowed base b, we’ll always have logb 1 = 0.
For the MCAT, the two most important bases are b = 10 and b = e. Base-10 logs are called common logs, and the “10” is often not written at all:
log y means log10 y
The base-10 log is useful because we use a decimal number system, which is based (pun intended) on the number 10. For example, the number 273.15 means (2 × 102) + (7 × 101) + (3 × 100) + (1 × 10−1) + (5 × 10−2). In physics, the formula for the decibel level of a sound uses the base-10 log. In chemistry, the base-10 log has many uses, such as finding values of the pH, pOH, pKa, and pKb.
Base-e logs are known as natural logs. Here, e is a particular constant, approximately equal to 2.7. This may seem like a strange number to choose as a base, but it makes calculus run smoothly—which is why it’s called the natural logarithm—because (and you don’t need to know this for the MCAT) the only numerical value of b for which the function f(x) = bx is its own derivative is b = e = 2.71828.… Base-e logs are often used in the mathematical description of physical processes in which the rate of change of some quantity is proportional to the quantity itself; radioactive decay is a typical example. The notation “ln” (the abbreviation, in reverse, for natural logarithm) is often used to mean loge:
ln y means loge y
The relationship between the base-10 log and the base-e log of a given number can be expressed as ln y ≈ 2.3 log y. For example, if y = 1000 = 103, then ln 1000 ≈ 2.3 log 1000 = 2.3 × 3 = 6.9. You may also find it useful to know the following approximate values:
log 2 ≈ 0.3 |
ln 2 ≈ 0.7 |
log 3 ≈ 0.5 |
ln 3 ≈ 1.1 |
log 5 ≈ 0.7 |
ln 5 ≈ 1.6 |
Example 15-1:
a) What is log3 9?
b) Find log5 (1/25).
c) Find log4 8.
d) What is the value of log16 4?
e) Given that log 5 ≈ 0.7, what’s log 500?
f) Given that log 2 ≈ 0.3, find log (2 × 10−6).
g) Given that log 2 ≈ 0.3 and log 3 × 0.5, find log (6 × 1023).
Solution:
a) log3 9 = x is the same as 3x = 9, from which we see that x = 2. So, log3 9 = 2.
b) log5 (1/25) = x is the same as 5x = 1/25 = 1/52 = 5−2, so x = —2. Therefore, log5 (1/25) = —2.
c) log4 8 = x is the same as 4x = 8. Since 4x = (22)x = 22x and 8 = 23, the equation 4x = 8 is the same as 22x = 23, so 2x = 3, which gives x = 3/2. Therefore, log4 8 = 3/2.
d) log16 4 = x is the same as 16x = 4. To find x, you might notice that the square root of 16 is 4, so 161/2 = 4, which means log16 4 = 1/2. Alternatively, we can write 16x as (42)x = 42x and 4 as 41. Therefore, the equation 16x = 4 is the same as 42x = 41, so 2x = 1, which gives x = 1/2.
e) log 500 = log (5 × 100) = log 5 + log 100, where we used Law 1 in the last step. Since log 100 = log 102 = 2, we find that log 500 ≈ 0.7 + 2 = 2.7.
f) log (2 × 10−6) = log 2 + log 10−6, by Law 1. Since log 10−6 = —6, we find that log (2 × 10−6) ≈ 0.3 + (—6) = —5.7.
g) log (6 × 1023) = log 2 + log 3 + log 1023, by Law 1. Since log 1023 = 23, we find that log (6 × 1023) ≈ 0.3 + 0.5 + 23 = 23.8.
Example 15-2: In each case, find y.
a) log2 y = 5
b) log2 y = —3
c) log y = 4
d) log y = 7.5
e) log y = —2.5
f) ln y = 3
Solution:
a) log2 y = 5 is the same as 25 = y, so y = 32.
b) log2 y = —3 is the same as 2−3 = y, which gives y = 1/23 = 1/8.
c) log y = 4 is the same as 104 = y, so y = 10,000.
d) log y = 7.5 is the same as 107.5 = y. We’ll rewrite 7.5 as 7 + 0.5, so y =107+(0.5) = 107 × 100.5. Because 100.5 = 101/2 = ![]() , which is approximately 3, we find that y ≈ 107 × 3 = 3 × 107.
, which is approximately 3, we find that y ≈ 107 × 3 = 3 × 107.
e) log y = —2.5 is the same as 10−2.5 = y. We’ll rewrite —2.5 as —3 + 0.5, so y = 10−3+(0.5) = 10−3 × 100.5. Because 100.5 = 101/2 = ![]() , which is approximately 3, we have that y ≈ 10−3 × 3 = 0.003.
, which is approximately 3, we have that y ≈ 10−3 × 3 = 0.003.
f) ln y = 3 means loge y = 3; this is the same as y = e3 (which is about 20).
Example 15-3: The definition of the pH of an aqueous solution is
pH = —log [H3O+] (or, simply, —log [H+])
where [H3O+] is the hydronium ion concentration (in M).
Part I: Find the pH of each of the following solutions:
a) coffee, with [H3O+] = 8 × 10−6 M
b) seawater, with [H3O+] = 3 × 10−9 M
c) vinegar, with [H3O+] = 1.3 × 10−3 M
Part II: Find [H3O+] for each of the following pH values:
d) pH = 7
e) pH = 11.5
f) pH = 4.7
Solution:
a) pH = —log (8 × 10−6) = —[log 8 + log (10−6)] = —log 8 + 6. We can now make a quick approximation by simply noticing that log 8 is a little less than log 10; that is, log 8 is a little less than 1. Let’s say it’s 0.9. Then pH ≈ —0.9 + 6 = 5.1.
b) pH = —log (3 × 10−9) = —[log 3 + log (10−9)] = —log 3 + 9. We now make a quick approximation by simply noticing that log 3 is about 0.5 (after all, 90.5 is 3, so 100.5 is close to 3). This gives pH ≈ —0.5 + 9 = 8.5.
c) pH = —log (1.3 × 10−3) = —[log 1.3 + log (10−3)] = —log 1.3 + 3. We can now make a quick approximation by simply noticing that log 1.3 is just a little more than log 1; that is, log 1.3 is a little more than 0. Let’s say it’s 0.1. This gives pH ≈ —0.1 + 3 = 2.9.
Note 1:
We can generalize these three calculations as follows: If [H3O+] = m × 10−n M, where 1 ≤ m < 10 and n is an integer, then the pH is between (n — 1) and n; it’s closer to (n — 1) if m > 3 and it’s closer to n if m < 3. (We use 3 as the cutoff since log 3 ≈ 0.5.)
d) If pH = 7, then —log [H3O+] = 7, so log [H3O+] = —7, which means [H3O+] = 10−7 M.
e) If pH = 11.5, then —log [H3O+] = 11.5, so log [H3O+] = —11.5, which means [H3O+] = 10−11.5 = 10(0.5)—12 = 100.5 × 10−12 ≈ 3 × 10−12 M.
f) If pH = 4.7, then —log [H3O+] = 4.7, so log [H3O+] = —4.7, which means [H3O+] = 10−4.7 = 10(0.3)—5 = 100.3 × 10−5 ≈ 2 × 10−5 M. [10−0.3 ≈ 2 follows from the fact that log 2 ≈ 0.3.]
Note 2:
We can generalize these last two calculations as follows: If pH = n.m, where n is an integer and m is a digit from 1 to 9, then [H3O+] = y × 10−(n+1) M, where y is closer to 1 if m > 3 and closer to 10 if m < 3. (We take y = 5 if m = 3.)
Example 15-4: The definition of the pKa of a weak acid is
pKa = —log Ka
where Ka is the acid’s ionization constant.
Part I: Approximate the pKa of each of the following acids:
a) HBrO, with Ka = 2 × 10−9
b) HNO2, with Ka = 7 × 10−4
c) HCN, with Ka = 6 × 10−10
Part II: Approximate Ka for each of the following pKa values:
d) pKa = 12.5
e) pKa = 2.7
f) pKa = 9.2
Solution:
a) pKa = —log (2 × 10−9) = —[log 2 + log (10−9)] = —log 2 + 9. We can now make a quick approximation by remembering that log 2 is about 0.3. Then pKa = —0.3 + 9 = 8.7. Because the formula to find pKa from Ka is exactly the same as the formula for finding pH from [H+], we could also make use of Note 1 in the solution to Example 15-3. If Ka = m´ × 10−n M, where 1 ≤ m < 10 and n is an integer, then the pKa is between (n — 1) and n; it’s closer to (n — 1) if m > 3 and it’s closer to n if m < 3. In this case, m = 2 and n = 9, so the pKa is between (n — 1) = 8 and n = 9. And, since 2 < 3, the pKa will be closer to 9 (which is just what we found, since we got the value 8.7). Given a list of possible choices for the pKa of this acid, just recognizing that it’s a little less than 9 will be sufficient.
b) With Ka = 7 × 10−4, we have m = 7 and n = 4. Therefore, the pKa will be between (n — 1) = 3 and n = 4. Since m = 7 is greater than 3, the value of pKa will be closer to 3 (around, say, 3.2).
c) With Ka = 6 × 10−10, we have m = 6 and n = 10. Therefore, the pKa will be between (n — 1) = 9 and n = 10. Since m = 6 is greater than 3, the value of pKa will be closer to 9 (around, say, 9.2).
d) If pKa = 12.5, then —log Ka = 12.5, so log Ka = —12.5, which means Ka = 10−12.5 = 10(0.5)—13 = 100.5 × 10−13 ≈ 3 × 10−13. We could also make use of Note 2 in the solution to Example 15-3. If pKa = n.m, where n is an integer and m is a digit from 1 to 9, then Ka = y × 10−(n+1) M, where y is closer to 1 if m > 3 and y is closer to 10 if m < 3. In this case, with pKa = 12.5, we have n = 12 and m = 5, so the Ka value is y × 10−(12+1) = y × 10−13, with y closer to 1 (than to 10) since m = 5 is greater than 3 (this agrees with what we found, since we calculated that Ka ≈ 3 × 10−13).
e) With pKa = 2.7, we have n = 2 and m = 7. Therefore, the Ka value is y × 10−(2+1) = y × 10−3, with y close to 1 since m = 7 is greater than 3. We can check this as follows: If pKa = 2.7, then —log Ka = 2.7, so log Ka = —2.7, which means Ka = 10−2.7 = 10(0.3)—3 = 100.3 × 10−3 ≈ 2 × 10−3.
f) With pKa = 9.2, we have n = 9 and m = 2. Therefore, the Ka value is y × 10−(9+1) = y × 10−10, with y closer to 10 (than to 1) since m = 2 is less than 3. We can say that Ka ≈ 6 × 10−10.
Example 15-5:
a) If y increases by a factor of 100, what happens to log y?
b) If y decreases by a factor of 1000, what happens to log y?
c) If y increases by a factor of 30,000, what happens to log y?
d) If y is reduced by 99%, what happens to log y?
Solution:
a) If y changes to y´ = 100y, then the log increases by 2, since
log y´ = log(100y) = log 100 + log y = log 102 + log y = 2 + log y
b) If y changes to y´ = y/1000, then the log decreases by 3, since
log y´ = log(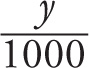 ) = log y − log 1000 = log y − log 103 = log y − 3
) = log y − log 1000 = log y − log 103 = log y − 3
c) If y changes to y´ = 30,000y, then the log increases by about 4.5, since
log y´ = log(30000 y) = log 3 + log 10000 + log y ≈ 0.5 + 4 + log y = 4.5 + log y
d) If y is reduced by 99%, that means we’re subtracting 0.99y from y, which leaves 0.01y = y/100. Therefore, y has decreased by a factor of 100. And if y changes to y´ = y/100, then the log decreases by 2, since
log y´ = log (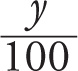 ) = log y − log 100 = log y − log 102 = log y − 2
) = log y − log 100 = log y − log 102 = log y − 2
Example 15-6: A radioactive substance has a half-life of 70 hours. For each of the fractions below, figure out how many hours will elapse until the amount of substance remaining is equal to the given fraction of the original amount.
a) 1/4
b) 1/8
c) 1/3
Solution:
a) After one half-life has elapsed, the amount remaining is 1/2 the original (by definition). After another half-life elapses, the amount remaining is now 1/2 of 1/2 the original amount, which is 1/4 the original amount. Therefore, a decrease to 1/4 the original amount requires 2 half-lives, which in this case is 2(70 hr) = 140 hr.
b) The fraction 1/8 is equal to 1/2 of 1/2 of 1/2; that is, 1/8 = (1/2)3. In terms of half-lives, a decrease to 1/8 the original amount requires 3 half-lives, which in this case is equal to 3(70 hr) = 210 hr. In general, a decrease to (1/2)n the original amount requires n half-lives.
c) The fraction 1/3 is not a whole-number power of 1/2, so we can’t directly apply the fact given in the italicized sentence in the solution to part (b). However, 1/3 is between 1/2 and 1/4, so the time to get to 1/3 the original amount is between 1 and 2 half-lives. Since one half-life is 70 hr, the amount of time is between 70 and 140 hours; the middle of this range (since 1/3 is roughly in the middle between 1/2 and 1/4) is about 110 hours. The most general formula for calculating the elapsed time involves a logarithm: If x < 1 is the fraction of a radioactive substance remaining after a time t has elapsed, then
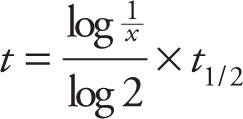
where t1/2 is the half-life. (If you want to use this formula, remember that log 2 ≈ 0.3.)