MCAT General Chemistry Review - Alexander Stone Macnow, MD 2019-2020
Solutions
Concentration
LEARNING GOALS
After Chapter 9.2, you will be able to:
· Calculate the molality, molarity, or normality of a compound in solution
· Apply MiVi = MfVf to calculate dilution of a solution
· Calculate mole fraction and percent composition by mass
Concentration denotes the amount of solute dissolved in a solvent. There are many different ways of expressing concentration, and different units have been standardized for specific everyday situations. For example, alcohol content in liquors like vodka, gin, or rum is expressed in volume percent (volume of solute divided by volume of solution times 100 percent). Alcoholic proof is twice the volume percent. The sugar content of orange juice and other fruit juices is measured in units of degrees Brix (°Bx), which is a mass percent: mass of glucose divided by mass of solution times 100 percent.
UNITS OF CONCENTRATION
On the MCAT, concentrations are commonly expressed as percent composition by mass, mole fraction, molarity, molality, and normality.
Percent Composition by Mass
The percent composition by mass is given by the equation
![]()
Equation 9.1
MCAT Expertise
It is important to have a good idea of how to work with all of these ways of expressing concentration because more than one may show up on Test Day.
Percent composition is used not only for aqueous solutions, but also for metal alloys and other solid-in-solid solutions.
Example:
What is the percent composition by mass of a salt water solution if 100 g of the solution contains 20 g of NaCl?
Solution:
![]()
=20% NaCl solution.
Mole Fraction
The mole fraction (X) of a compound is given by the equation
![]()
Equation 9.2
The sum of the mole fractions in a system will always equal 1. The mole fraction is used to calculate the vapor pressure depression of a solution, described later in this chapter, as well as the partial pressures of gases in a system, described in Chapter 8 of MCAT General Chemistry Review.
Example:
If 184 g glycerol (C3H8O3) is mixed with 180 g water, what will be the mole fractions of the two components? (Note: Molar mass of ![]() molar mass of
molar mass of ![]() )
)
Solution:
First, determine the number of moles of each compound:
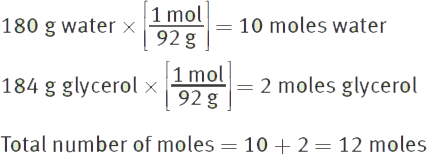
Then, determine the mole fractions:
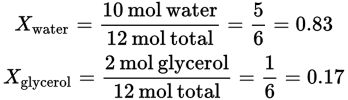
Molarity
The molarity (M) of a solution is defined as
![]()
Equation 9.3
Solution concentrations are usually expressed in terms of molarity, and this is the most common unit for concentration on the MCAT. Unless otherwise specified, representations of concentration using brackets—such as [Na+]—indicate molarity. Note that the volume term in the denominator of molarity refers to the solution volume, not the volume of solvent used to prepare the solution—although the two values are often close enough to approximate the solution volume using the solvent volume. Molarity is used for rate laws, the law of mass action, osmotic pressure, pH and pOH, and the Nernst equation.
MCAT Expertise
Note that for dilute solutions, the volume of the solution is approximately equal to the volume of solvent used, which simplifies our calculations on Test Day. However, technical questions could ask you to distinguish between these two. For example, when you add two kilograms of sucrose (table sugar) to a liter of water at room temperature (achieving saturation), the volume of solution is certainly larger than 1 L!
Example:
If enough water is added to 11 g of CaCl2 to make 100 mL of solution, what is the molarity of the solution?
Solution:
First, calculate the number of moles of CaCl2:
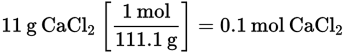
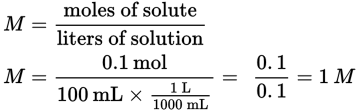
Then determine the molarity:
Molality
The molality (m) of a solution is defined as
![]()
Equation 9.4
For dilute aqueous solutions at 25°C, the molality is approximately equal to molarity because the density of water at this temperature is 1 kilogram per liter. However, note that this is an approximation and true only for dilute aqueous solutions. As aqueous solutions become more concentrated with solute, their densities become significantly different from that of pure water; most water-soluble solutes have molar masses significantly greater than that of water, so the density of the solution increases as the concentration increases. You won’t use molality very often, so be mindful of the special situations when it is required: boiling point elevation and freezing point depression.
Example:
If 10 g NaOH are dissolved in 500 g of water, what is the molality of the solution?
Solution:
First, calculate the number of moles of NaOH:
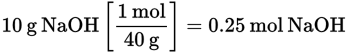
Then determine the molality:

Normality
We discussed the related concepts of gram equivalent weight, equivalents, and normality in Chapter 4 of MCAT General Chemistry Review. The normality (N) of a solution is equal to the number of equivalents of interest per liter of solution. An equivalent is a measure of the reactive capacity of a molecule. Most simply, an equivalent is equal to a mole of the species of interest—protons, hydroxide ions, electrons, or ions.
To calculate the normality of a solution, we need to know what purpose the solution serves because it is the concentration of the reactive species with which we are concerned. For example, in acid—base reactions, we are most concerned with the concentration of hydrogen ions; in oxidation—reduction reactions, we are most concerned with the concentration of electrons. Normality is unique among concentration units in that it is reaction dependent. For example, in acidic solution, 1 mole of the permanganate ion (MnO4−) will readily accept 5 moles of electrons, so a 1 M solution would be 5 N. However, in alkaline solution, 1 mole of permanganate will accept only 1 mole of electrons, so in alkaline solution, a 1 M permanganate solution would be 1 N.
MCAT Expertise
Simple ideas on Test Day will make things easier. So, when you come across normality, think of it as molarity of the stuff of interest in the reaction.
DILUTION
A solution is diluted when solvent is added to a solution of higher concentration to produce a solution of lower concentration. The concentration of a solution after dilution can be determined using the equation
MiVi = MfVf
Equation 9.5
where M is molarity, V is volume, and the subscripts i and f refer to the initial and final values, respectively.
MCAT Expertise
Though not a unique means of measuring concentration or dilution, the term parts-per can be used to indicate concentration of a dissolved substance in a solution (most commonly water). Parts-per-million (ppm, 10—6) is the most common usage. If a problem states there is one ppm of substance X in water, that would indicate there is 1 mg/L of water, as there would be 1 millionth of a gram per gram of water, and the density of water is 1 g/mL. On Test Day, prior to converting from ppm, make sure to assess whether conversion to a different unit of measure is actually required, as this conversion can typically be avoided on the MCAT.
Example:
A chemist wishes to prepare 300 mL of a 1.1 M NaOH solution from a 5.5 M NaOH stock solution. What volume of stock solution should be diluted with pure water to obtain the desired solution?
Solution:
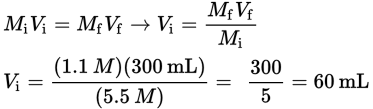
Note that one can use mL or L in the equation, as long as the units are consistent.
MCAT Expertise
This equation is worthy of memorization. Note that a similar equation is used for the equivalence point in acid—base chemistry, as discussed in Chapter 10 of MCAT General Chemistry Review.
MCAT Concept Check 9.2:
Before you move on, assess your understanding of the material with these questions.
1. If you mix 180 g of the following compounds in 250 mL of water ![]() what are their concentrations in molality, molarity, and normality (for acid—base chemistry)?
what are their concentrations in molality, molarity, and normality (for acid—base chemistry)?
|
Compound |
Molality |
Molarity |
Normality |
|
Glucose |
|||
|
Carbonate |
2. You are working in a sewage treatment facility and are assaying chlorine in a water sample. You need to dilute the water sample from 100 ppm stock to 25 ppm and create 100 mL of solution. Calculate the amount of stock solution needed and determine how you would create your final solution:
3. A stock solution for making typical IV saline bags contains 90.0 g of NaCl per 10 liters of water ![]() What is the mole fraction and the percent composition by mass of NaCl in the saline solutions?
What is the mole fraction and the percent composition by mass of NaCl in the saline solutions?
o Mole fraction:
o Percent composition by mass: