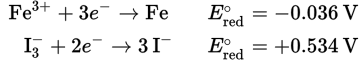MCAT General Chemistry Review - Alexander Stone Macnow, MD 2019-2020
Electrochemistry
Cell Potentials
LEARNING GOALS
After Chapter 12.2, you will be able to:
· Describe how standard reduction potentials are measured
· Explain the importance of the sign for electromotive force
· Determine whether a cell using a given reaction is galvanic or electrolytic
· Calculate the net E value for a redox reaction between two species:
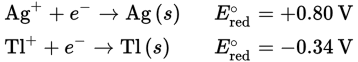
For galvanic cells, the direction of spontaneous movement of charge is from the anode, the site of oxidation, to the cathode, the site of reduction. This is simple enough to remember, but it begs the question: how do we determine which electrode species will be oxidized and which will be reduced? The relative tendencies of different chemical species to be reduced have been determined experimentally, using the tendency of the hydrogen ion (H+) to be reduced as an arbitrary zero reference point.
REDUCTION POTENTIALS
A reduction potential is measured in volts (V) and defined relative to the standard hydrogen electrode (SHE), which is given a potential of 0 V by convention. The species in a reaction that will be oxidized or reduced can be determined from the reduction potential of each species, defined as the tendency of a species to gain electrons and to be reduced. Each species has its own intrinsic reduction potential; the more positive the potential, the greater the tendency to be reduced.
Standard reduction potential (E°red) is measured under standard conditions: 25°C (298 K), 1 atm pressure, and 1 M concentrations. The relative reactivities of different half-cells can be compared to predict the direction of electron flow. A more positive E°red means a greater relative tendency for reduction to occur, while a less positive E°red means a greater relative tendency for oxidation to occur.
Key Concept
A reduction potential is exactly what it sounds like. It tells us how likely a compound is to be reduced. The more positive the value, the more likely it is to be reduced—the more it wants to be reduced.
For galvanic cells, the electrode with the more positive reduction potential is the cathode, and the electrode with the less positive reduction potential is the anode. Because the species with a stronger tendency to gain electrons (that wants to gain electrons more) is actually doing so, the reaction is spontaneous and ΔG is negative. For electrolytic cells, the electrode with the more positive reduction potential is forced by the external voltage source to be oxidized and is, therefore, the anode. The electrode with the less positive reduction potential is forced to be reduced and is, therefore, the cathode. Because the movement of electrons is in the direction against the tendency or desires of the respective electrochemical species, the reaction is nonspontaneous and ΔG is positive.
Example:
Given the following half-reactions and E°red values, determine which species would be oxidized and which would be reduced in a galvanic cell.
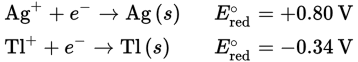
Solution:
E°red indicates the reduction potential, or the likelihood of a compound to be reduced via a given reaction. A positive E°red value indicates a spontaneous reduction, and a negative value indicates a non—spontaneous reduction. In a galvanic cell, Ag+ will be spontaneously reduced to Ag (s) and Tl (s) will be spontaneously oxidized to Tl+ because Ag+ has the more positive E°red and thus the more favorable reduction reaction. Therefore, the net ionic equation would be:
Ag+ + Tl (s) → Tl+ + Ag (s)
which is the sum of the two spontaneous half-reactions.
It should be noted that reduction and oxidation are opposite processes. Therefore, to obtain the oxidation potential of a given half-reaction, both the reduction half-reaction and the sign of the reduction potential are reversed. For instance, from the example above, the oxidation half-reaction and oxidation potential of Tl (s) are:
![]()
Note that, in the examples of batteries given above (lead—acid storage batteries and nickel—cadmium batteries), the oxidation half-reaction was given with the reduction potential of the reverse reaction. These two quantities have equal magnitudes but opposite signs. On the MCAT, reduction potentials are generally given rather than oxidation potentials. Therefore, all references in this book (with exception of the thallium example immediately above) are given using reduction potentials—not oxidation potentials.
THE ELECTROMOTIVE FORCE
Standard reduction potentials are also used to calculate the standard electromotive force (emf or E°cell) of a reaction, which is the difference in potential (voltage) between two half-cells under standard conditions. The emf of a reaction is determined by calculating the difference in reduction potentials between the two half-cells:
E°cell = E°red,cathode − E°red,anode
Equation 12.3
When subtracting standard potentials, do not multiply them by the number of moles oxidized or reduced. This is because the potential of each electrode does not depend on the size of the electrode (the amount of material), but rather the identity of the material. The standard reduction potential of an electrode will not change unless the chemical identity of that electrode is changed.
Key Concept
If you need to multiply each half-reaction by a common denominator to cancel out electrons when coming up with the net ionic equation, do not multiply the reduction potential, E°red, by that number. That would indicate a change in the chemical identity of the electrode, which is not occurring.
Example:
Given that the standard reduction potentials for Sm3+ and [RhCl6]3— are —2.41 V and +0.44 V, respectively, calculate the electromotive force of the following reaction:
Sm3+ + Rh + 6 Cl— → [RhCl6]3— + Sm
Solution:
First, determine the oxidation and reduction half-reactions. As written, the Rh is oxidized, and the Sm3+ is reduced:
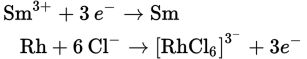
Now, we simply take the difference between the samarium(III) reduction potential and the hexachlororhodate(III) reduction potential. We need not change the sign on the hexachlororhodate(III) reduction potential because we are subtracting it from that of samarium(III).
Using the equation provided, the emf can be calculated as: —2.41 V — (+0.44 V) = —2.85 V. The cell is thus electrolytic. If this were instead a galvanic cell the reaction would proceed spontaneously to the left, toward reactants, in which case the Sm would be oxidized while [RhCl6]3— would be reduced with an emf of +2.85 V.
MCAT Concept Check 12.2:
Before you move on, assess your understanding of the material with these questions.
1. How are standard reduction potentials measured?
2. If a cell’s electromotive force (emf) is denoted as a positive value, what does that mean? What if it is negative?
o Positive emf:
o Negative emf:
3. Given the following reactions, determine whether the cell is galvanic or electrolytic:
o 2 Fe3+ (aq) + 2 Cl— (aq) → 2 Fe2+ (aq) + Cl2 (g) (E°cell = —0.59 V):
o 2 Fe3+ (aq) + 2 I— (aq) → 2 Fe2+ (aq) + I2 (aq) (E°cell = +0.25 V):
4. Given the two half-reactions below, what would be the spontaneous oxidation—reduction reaction between these two species?