MCAT General Chemistry Review - Alexander Stone Macnow, MD 2019-2020
The Periodic Table
Periodic Properties of the Elements
LEARNING GOALS
After Chapter 2.3, you will be able to:
· Compare the atomic radius of neutral atoms to their ions
· Rank elements by ionization energy, electron affinity, electronegativity, or atomic radius: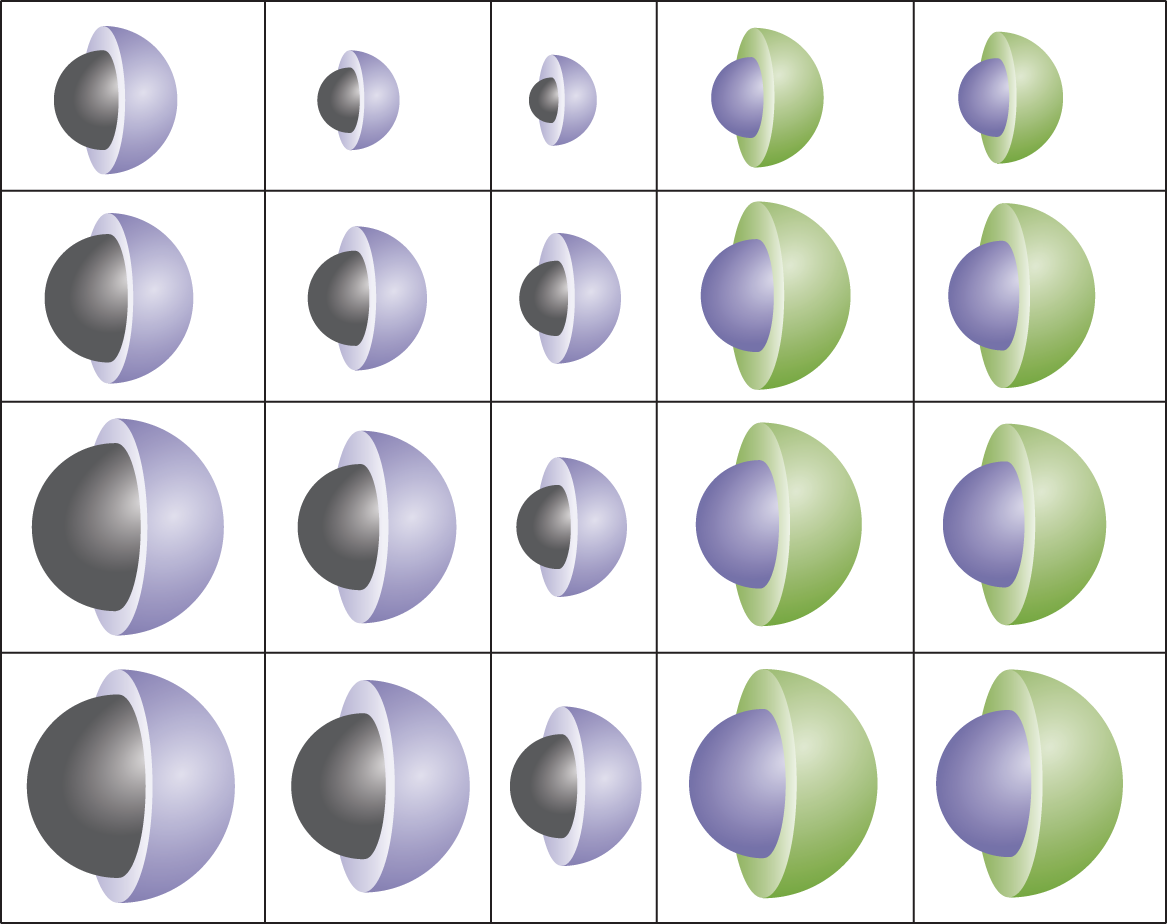
MCAT Expertise
The “High-Yield” badge on this section indicates that the content is frequently tested on the MCAT.
The MCAT does not expect you to have memorized the entire periodic table. Fortunately, the periodic table is a guide unto itself, a self-referencing localization system for all of the elements. Remember, the modern table is organized in such a way to represent visually the periodicity of chemical and physical properties of the elements. The periodic table, then, can provide you with a tremendous amount of information that otherwise would have to be memorized. Note, though, that while you do not need to memorize the periodic table for the MCAT, you do need to understand the trends within the periodic table that help predict the chemical and physical behaviors of the elements.
MCAT Expertise
Don’t try to memorize the periodic table. You will have access to it on Test Day through the test interface. Do understand its configuration and trends so that you can use it efficiently to get a higher score!
Before exploring the periodic trends, let’s take stock of three key rules that control how valence electrons work in an atom. First, as we’ve already mentioned, as one moves from left to right across a period, electrons and protons are added one at a time. As the positivity of the nucleus increases, the electrons surrounding the nucleus, including those in the valence shell, experience a stronger electrostatic pull toward the center of the atom. This causes the electron cloud, which is the outer boundary defined by the valence shell electrons, to move closer and bind more tightly to the nucleus. This electrostatic attraction between the valence shell electrons and the nucleus is known as the effective nuclear charge (Zeff), a measure of the net positive charge experienced by the outermost electrons. This pull is somewhat mitigated by nonvalence electrons that reside closer to the nucleus. For elements in the same period, Zeff increases from left to right. The parts of an atom responsible for Zeff are illustrated in Figure 2.4.
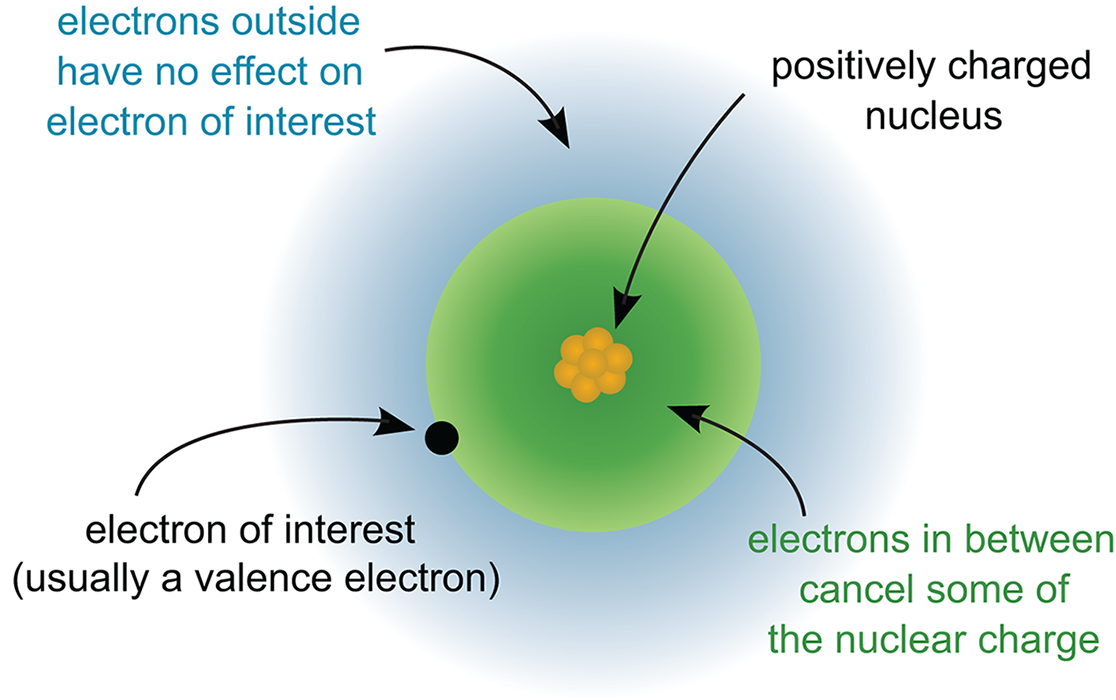 Figure 2.4. Factors that Determine Effective Nuclear Charge (Zeff)
Figure 2.4. Factors that Determine Effective Nuclear Charge (Zeff)
Bridge
Zeff relies on the principles of electrostatic forces defined in Chapter 5 of MCAT Physics and Math Review. The values q1 and q2 can represent the net charge of the nucleus and valence electron shell, respectively. The larger each charge gets (going to the right in the periodic table), the higher the value of Zeff.
Second, as one moves down the elements of a given group, the principal quantum number increases by one each time. This means that the valence electrons are increasingly separated from the nucleus by a greater number of filled principal energy levels, which can also be called inner shells. The result of this increased separation is a reduction in the electrostatic attraction between the valence electrons and the positively charged nucleus. These outermost electrons are held less tightly as the principal quantum number increases. As one goes down in a group, the increased shielding created by the inner shell electrons cancels the increased positivity of the nucleus. Thus, the Zeff is more or less constant among the elements within a given group. Despite this fact, the valence electrons are held less tightly to the nucleus as one moves down a group due to the increased separation between valence electrons and the nucleus.
Third, elements can also gain or lose electrons in order to achieve a stable octet formation representative of the noble (inert) gases (Group VIIIA or Group 18). In Chapter 3 of MCAT General Chemistry Review, we will discuss how the octet rule is hardly a rule at all because there are many exceptions. For now, keep in mind that elements, especially the ones that have biological roles, tend to be most stable with eight electrons in their valence shell.
These three facts are guiding principles as we work toward an understanding of the trends demonstrated in the periodic table. In fact, the trend for effective nuclear charge across a period and the impact of increasing the number of inner shells down a group will help derive all the trends we discuss below.
ATOMIC AND IONIC RADII
Think of an atom as a cloud of electrons surrounding a dense core of protons and neutrons. The atomic radius of an element is thus equal to one-half of the distance between the centers of two atoms of an element that are briefly in contact with each other. The distance between two centers of circles in contact is akin to a diameter, making this radius calculation simple. The atomic radius cannot be measured by examining a single atom because the electrons are constantly moving around, making it impossible to mark the outer boundary of the electron cloud.
Key Concept
Atomic radius refers to the size of a neutral element, while an ionic radius is dependent on how the element ionizes based on its element type and group number.
As we move across a period from left to right, protons and electrons are added one at a time to the atoms. Because the electrons are being added only to the outermost shell and the number of inner-shell electrons remains constant, the increasing positive charge of the nucleus pulls the outer electrons more closely inward and holds them more tightly. The Zeff increases left to right across a period, and as a result, atomic radius decreases from left to right across a period.
MCAT Expertise
Atomic radius is essentially opposite that of all other periodic trends. While others increase going up and to the right, atomic radius increases going down and to the left.
As we move down a group, the increasing principal quantum number implies that the valence electrons will be found farther away from the nucleus because the number of inner shells is increasing, separating the valence shell from the nucleus. Although the Zeff remains essentially constant, the atomic radius increases down a group. Within each group, the largest atom will be at the bottom, and within each period, the largest atom will be in Group IA (Group 1). For reference, the largest atomic radius in the periodic table belongs to cesium (Cs, 260 pm), and the smallest belongs to helium (He, 25 pm). Francium is typically not considered because it is exceptionally rare in nature. Figure 2.5 displays a graph of atomic radius vs. atomic number, with Group IA elements possessing the largest atomic radius in each row.
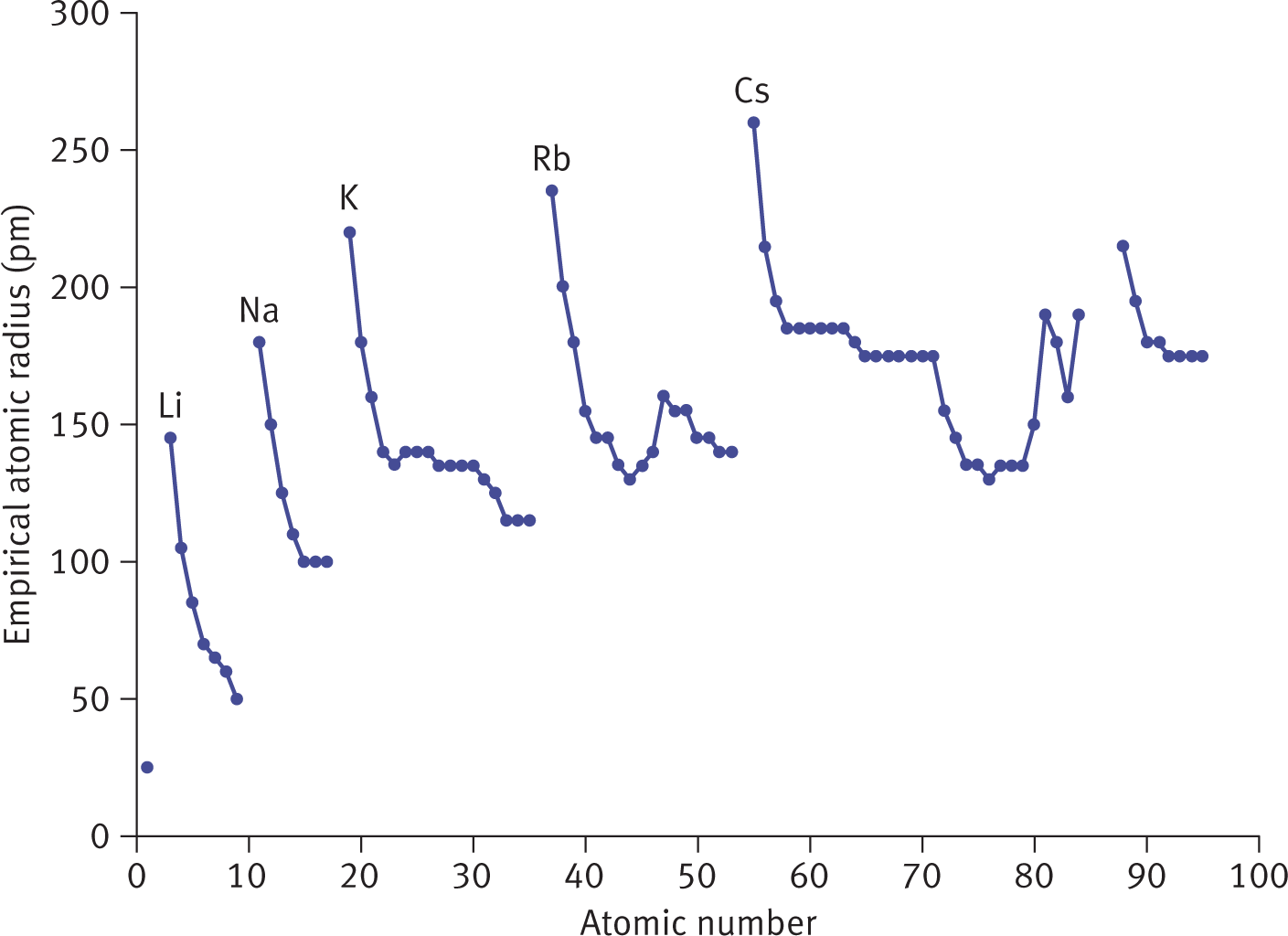 Figure 2.5. Atomic Radius (in pm) vs. Atomic Number
Figure 2.5. Atomic Radius (in pm) vs. Atomic Number
Unlike atomic radii, ionic radii will require some critical thinking and periodic table geography to determine. In order to understand ionic radii, we must make two generalizations. One is that metals lose electrons and become positive, while nonmetals gain electrons and become negative. The other is that metalloids can go in either direction, but tend to follow the trend based on which side of the metalloid line they fall on. Thus, silicon (Si) behaves more like a nonmetal, while germanium (Ge) tends to act more like a metal. On the MCAT, these generalizations can also be inferred from information found in passages and questions, such as oxidation states in compounds.
For nonmetals close to the metalloid line, their group number dictates that they require more electrons than other nonmetals to achieve the electronic configuration seen in Group VIIIA (Group 18). These nonmetals gain electrons while their nuclei maintain the same charge. Therefore, these nonmetals close to the metalloid line possess a larger ionic radius than their counterparts closer to Group VIIIA.
For metals, the trend is similar but opposite. Metals closer to the metalloid line have more electrons to lose to achieve the electronic configuration seen in Group VIIIA. Because of this, the ionic radius of metals near the metalloid line is dramatically smaller than that of other metals. Metals closer to Group IA have fewer electrons to lose and therefore experience a less drastic reduction in radius during ionization. These changes are illustrated in Figure 2.6. Note that tellurium (Te) behaves as a nonmetal and boron (B) behaves as a metal; under varying conditions, these metalloids can have opposite behavior.
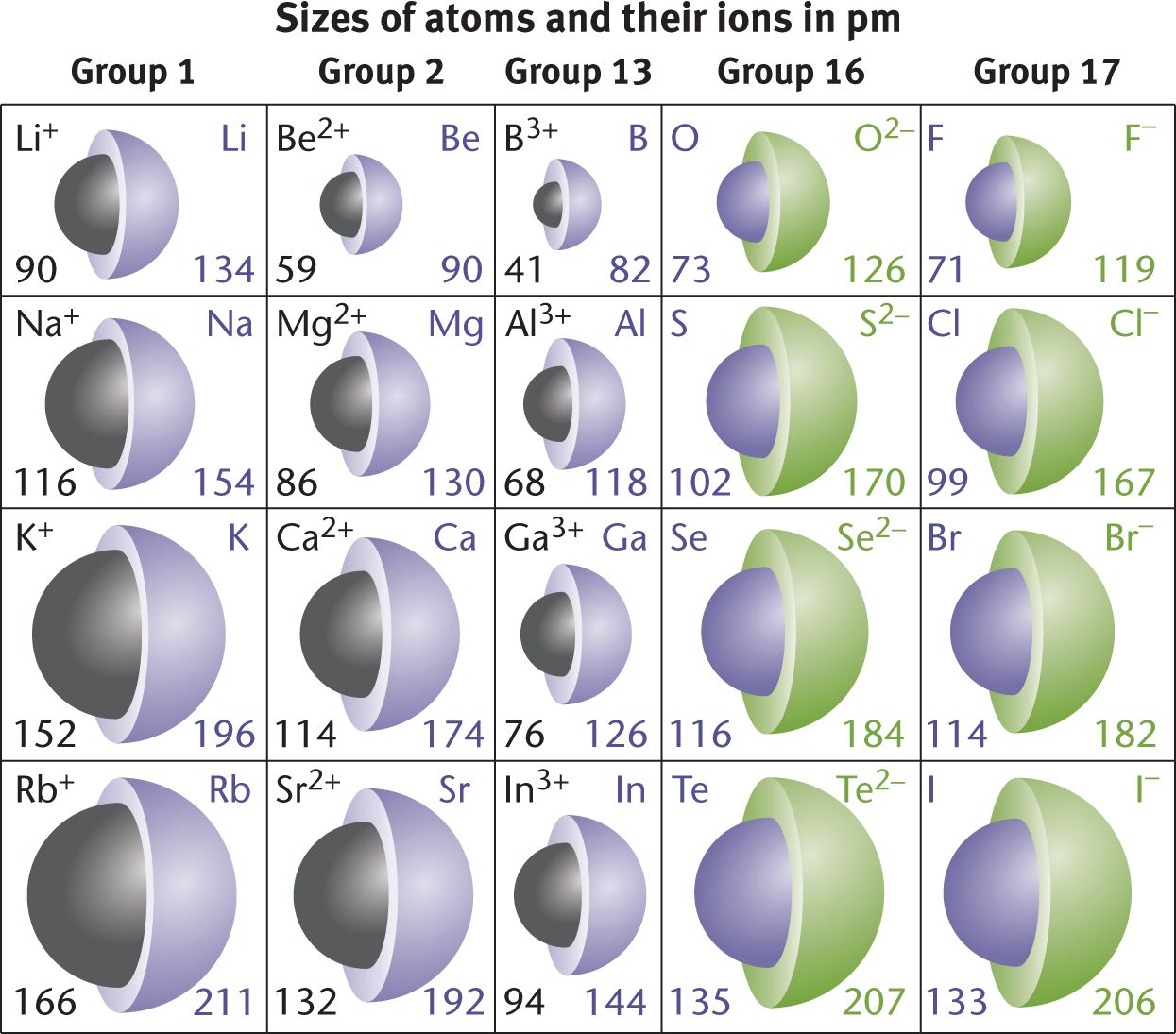 Figure 2.6. Ionic Radii (in pm) for Various Metals and NonmetalsNeutral atoms are shown in purple; cations in black; anions in green.
Figure 2.6. Ionic Radii (in pm) for Various Metals and NonmetalsNeutral atoms are shown in purple; cations in black; anions in green.
IONIZATION ENERGY
Ionization energy (IE), also known as ionization potential, is the energy required to remove an electron from a gaseous species. Removing an electron from an atom always requires an input of heat, which makes it an endothermic process. The greater the atom’s Zeff or the closer the valence electrons are to the nucleus, the more tightly bound they are. This makes it more difficult to remove one or more electrons, increasing the ionization energy. Thus, ionization energy increases from left to right across a period and from bottom to top in a group. The subsequent removal of a second or third electron requires increasing amounts of energy because the removal of more than one electron means that the electrons are being removed from an increasingly cationic (positive) species. The energy necessary to remove the first electron is called the first ionization energy; the energy necessary to remove the second electron from the univalent cation (X+) to form the divalent cation (X2+) is called the second ionization energy, and so on. For example:
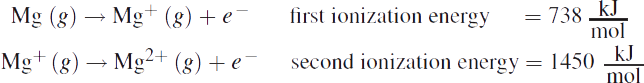
Elements in Groups IA and IIA (Groups 1 and 2), such as lithium and beryllium, have such low ionization energies that they are called the active metals. The active metals do not exist naturally in their neutral forms; they are always found in ionic compounds, minerals, or ores. The loss of one electron from the alkali metals (Group IA) or the loss of two electrons from the alkaline earth metals (Group IIA) results in the formation of a stable, filled valence shell. In contrast, the Group VIIA (Group 17) elements—the halogens—do not typically give up their electrons. In fact, in their ionic form, they are generally anions. The first ionization energies of the elements are shown in Figure 2.7.
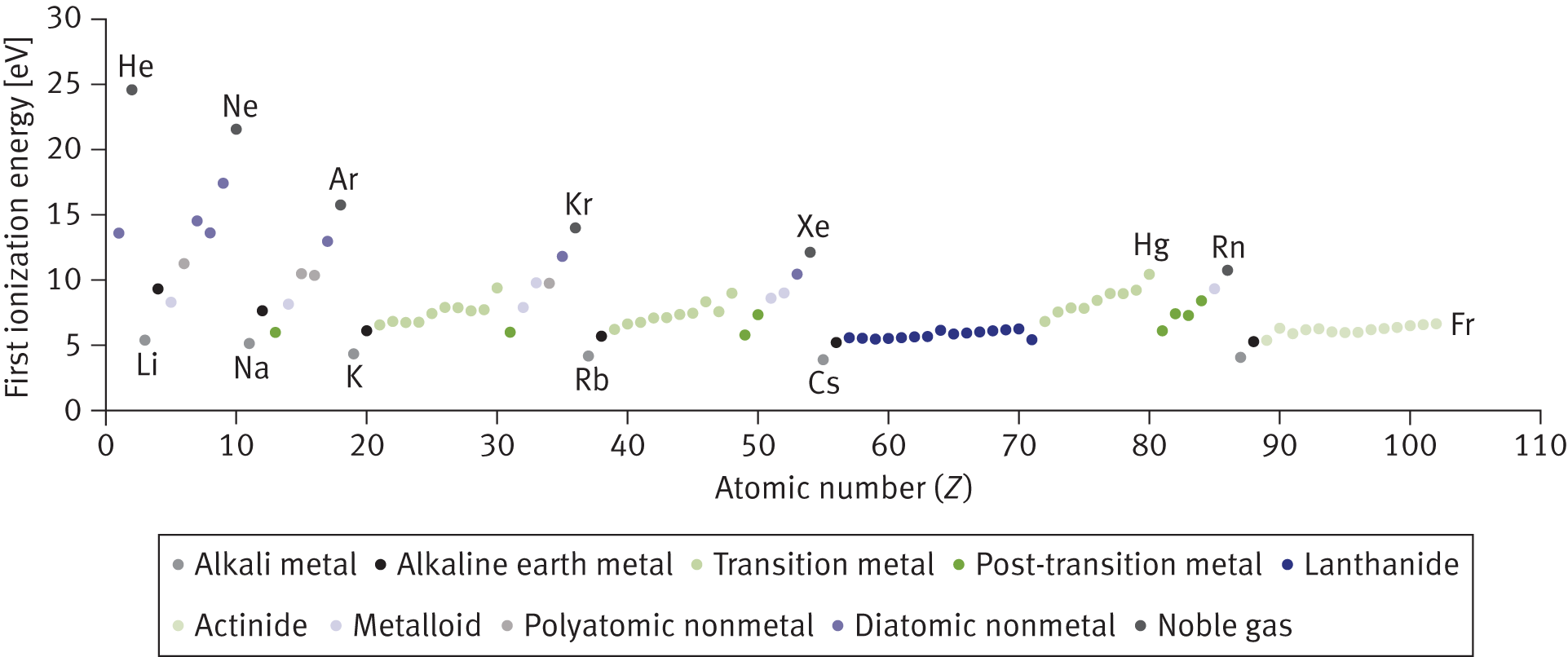 Figure 2.7. First Ionization Energies (in eV) of the Elements
Figure 2.7. First Ionization Energies (in eV) of the Elements
MCAT Expertise
First ionization energy (IE) will always be smaller than second IE, which will always be smaller than third IE. However, the degree to which the IE increases provides clues about the identity of the atom. If losing a certain number of electrons gives an element a noble gas-like electron configuration, then removing a subsequent electron will cost much more energy. For example
· Mg2+ (g) → Mg3+ (g) + e—
· ![]()
The values for second ionization energies are disproportionally larger for Group IA monovalent cations (like Na+) but generally not that much larger for Group IIA or subsequent monovalent cations (like Mg+). This is because removing one electron from a Group IA metal results in a noble gas-like electron configuration.
Group VIIIA (Group 18) elements, or noble or inert gases, are the least likely to give up electrons. They already have a stable electron configuration and are unwilling to disrupt that stability by giving up an electron. Therefore, noble gases are among the elements with the highest ionization energies.
ELECTRON AFFINITY
Halogens are the most “greedy” group of elements on the periodic table when it comes to electrons. By acquiring one additional electron, a halogen is able to complete its octet and achieve a noble gas configuration. This exothermic process expels energy in the form of heat. Electron affinity refers to the energy dissipated by a gaseous species when it gains an electron. Note the electron affinity is essentially the opposite concept from ionization energy. Because this is an exothermic process, ΔHrxn has a negative sign; however, the electron affinity is reported as a positive number. This is because electron affinity refers to the energy dissipated: if ![]() of energy is released,
of energy is released, ![]() and the electron affinity is
and the electron affinity is ![]() The stronger the electrostatic pull (the higher the Zeff) between the nucleus and the valence shell electrons, the greater the energy release will be when the atom gains the electron. Thus, electron affinity increases across a period from left to right. Because the valence shell is farther away from the nucleus as the principal quantum number increases, electron affinity decreases in a group from top to bottom. Groups IA and IIA (Groups 1 and 2) have very low electron affinities, preferring to give up electrons to achieve the octet configuration of the noble gas in the previous period. Conversely, Group VIIA (Group 17) elements have very high electron affinities because they need to gain only one electron to achieve the octet configuration of the noble gases (Group VIIIA or Group 18) in the same period. Although the noble gases would be predicted to have the highest electron affinities according to the trend, they actually have electron affinities on the order of zero because they already possess a stable octet and cannot readily accept an electron. Most metals also have low electron affinity values, as can be seen in Figure 2.8.
The stronger the electrostatic pull (the higher the Zeff) between the nucleus and the valence shell electrons, the greater the energy release will be when the atom gains the electron. Thus, electron affinity increases across a period from left to right. Because the valence shell is farther away from the nucleus as the principal quantum number increases, electron affinity decreases in a group from top to bottom. Groups IA and IIA (Groups 1 and 2) have very low electron affinities, preferring to give up electrons to achieve the octet configuration of the noble gas in the previous period. Conversely, Group VIIA (Group 17) elements have very high electron affinities because they need to gain only one electron to achieve the octet configuration of the noble gases (Group VIIIA or Group 18) in the same period. Although the noble gases would be predicted to have the highest electron affinities according to the trend, they actually have electron affinities on the order of zero because they already possess a stable octet and cannot readily accept an electron. Most metals also have low electron affinity values, as can be seen in Figure 2.8.
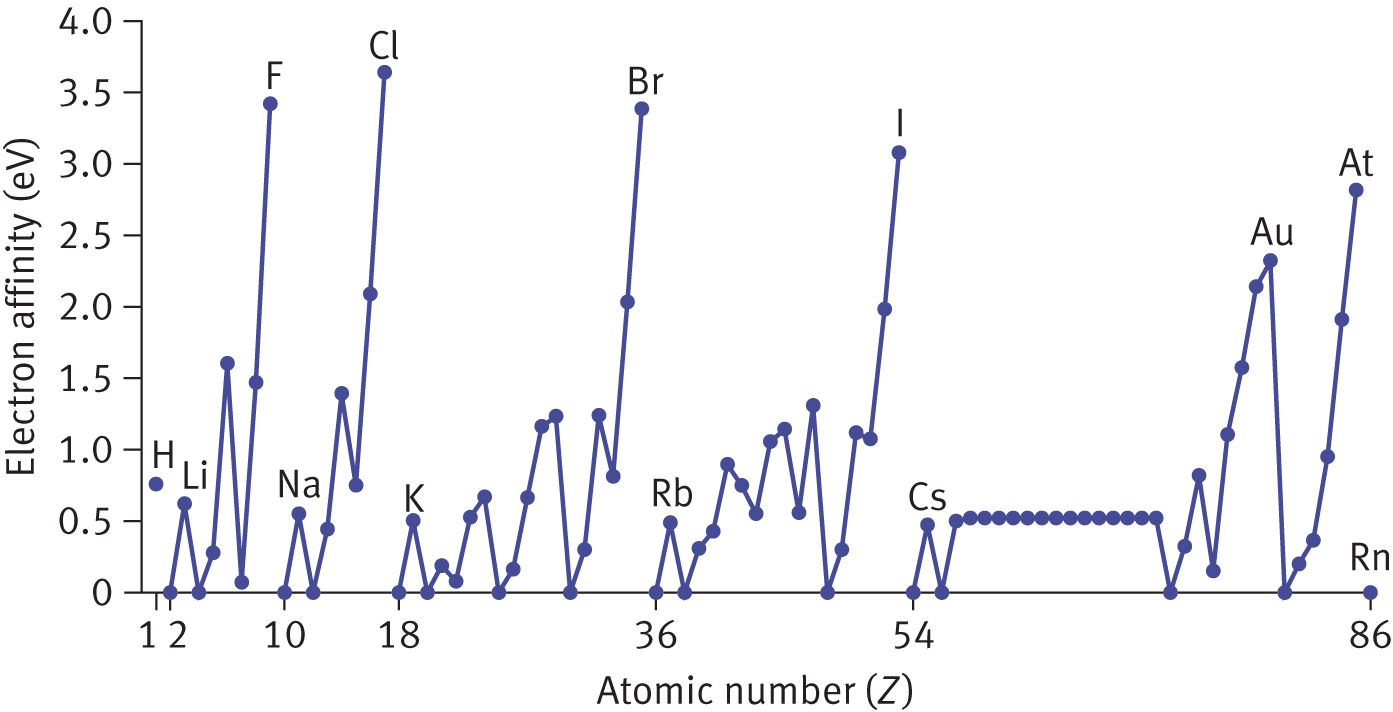 Figure 2.8. Electron Affinities (in eV) of the Elements
Figure 2.8. Electron Affinities (in eV) of the Elements
ELECTRONEGATIVITY
Electronegativity is a measure of the attractive force that an atom will exert on an electron in a chemical bond. The greater the electronegativity of an atom, the more it attracts electrons within a bond. Electronegativity values are related to ionization energies: the lower the ionization energy, the lower the electronegativity; the higher the ionization energy, the higher the electronegativity. The first three noble gases are exceptions: despite their high ionization energies, these elements have negligible electronegativity because they do not often form bonds.
The electronegativity value is a relative measure, and there are different scales used to express it. The most common scale is the Pauling electronegativity scale, which ranges from 0.7 for cesium, the least electronegative (most electropositive) element, to 4.0 for fluorine, the most electronegative element. Electronegativity increases across a period from left to right and decreases in a group from top to bottom. Figure 2.9 shows the electronegativity values of the elements.
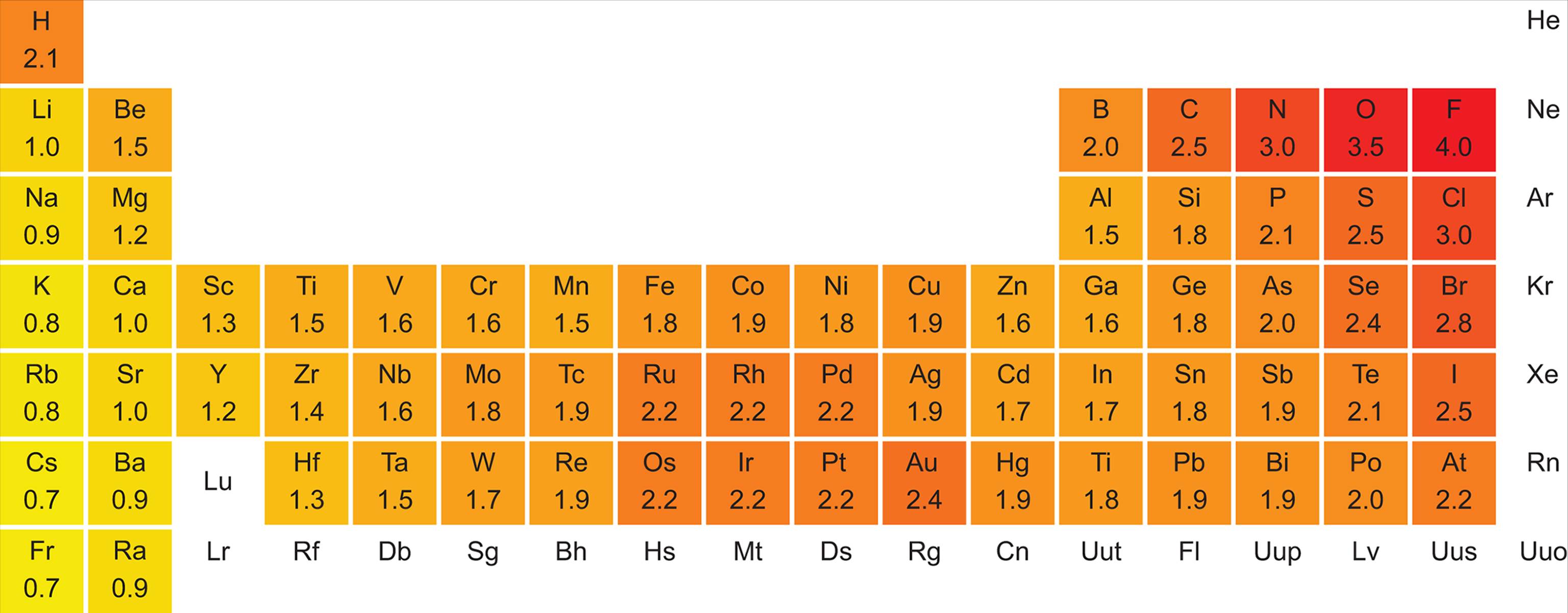 Figure 2.9. Pauling Electronegativity Values of the Elements
Figure 2.9. Pauling Electronegativity Values of the Elements
Key Concept
· Cs = largest, least electronegative, lowest ionization energy, least exothermic (lowest) electron affinity
· F = smallest, most electronegative, highest ionization energy, most exothermic (highest) electron affinity
The periodic trends are summarized together in Figure 2.10.
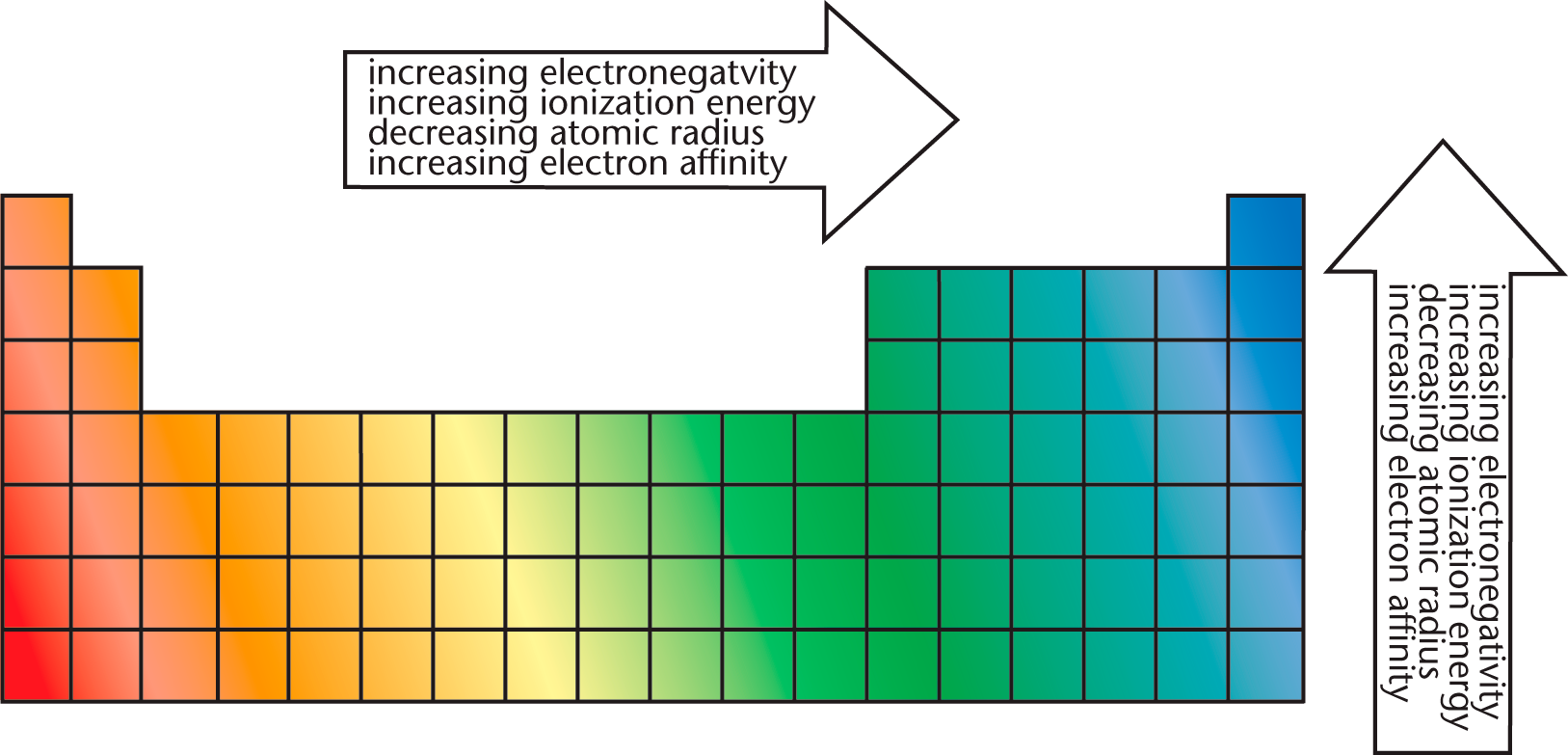 Figure 2.10. Periodic Trends
Figure 2.10. Periodic Trends
Key Concept
· Periodic Trends
· Left → Right
o Atomic radius ↓
o Ionization energy ↑
o Electron affinity ↑
o Electronegativity ↑
· Top → Bottom
o Atomic radius ↑
o Ionization energy ↓
o Electron affinity ↓
o Electronegativity ↓
Note: Atomic radius is always opposite the other trends. Ionic radius is variable.
MCAT Concept Check 2.3:
Before you move on, assess your understanding of the material with these questions.
1. In each of the following pairs, which has the larger radius?:
o F or F—
o K or K+
2. Rank the following elements by decreasing first ionization energy: calcium (Ca), carbon (C), germanium (Ge), potassium (K)
o
o
o
o
3. Rank the following elements by increasing electron affinity: barium (Ba), copper (Cu), sulfur (S), yttrium (Y)
o
o
o
o
4. Rank the following elements by decreasing electronegativity: antimony (Sb), neon (Ne), oxygen (O), thallium (Tl)
o
o
o
o
5. Rank the following elements by increasing atomic radius: niobium (Nb), praseodymium (Pr), tantalum (Ta), xenon (Xe)
o
o
o
o