MCAT General Chemistry Review - Alexander Stone Macnow, MD 2019-2020
The Periodic Table
The Chemistry of Groups
LEARNING GOALS
After Chapter 2.4, you will be able to:
· Identify the groups on the periodic table by the properties they exhibit
· Connect periodic table groups 1, 2, 16, 17, 18, and 3—12 to their common names
What follows is a discussion of the major groups you are likely to encounter on the MCAT. While it is rare to be tested on every group, it is important to understand the overarching trends we have already discussed and how they relate across different groups.
ALKALI METALS (IA)
The alkali metals (Group IA or Group 1) possess most of the classic physical properties of metals, except that their densities are lower than those of other metals (as described for lithium earlier in this chapter). The alkali metals have only one loosely bound electron in their outermost shells. Their Zeff values are very low, giving them the largest atomic radii of all the elements in their respective periods. This low Zeff value also explains the other trends: low ionization energies, low electron affinities, and low electronegativities. Alkali metals easily lose one electron to form univalent cations, and they react readily with nonmetals—especially the halogens—as in NaCl. Figure 2.11 illustrates the reaction of an alkali metal with water, a stereotypically violent reaction.
 Figure 2.11. Reaction of Sodium with WaterGroup IA metals react violently with water, forming strong bases.
Figure 2.11. Reaction of Sodium with WaterGroup IA metals react violently with water, forming strong bases.
Real World
Due to their high reactivity with water and air, most alkali metals are stored in mineral oil.
ALKALINE EARTH METALS (IIA)
The alkaline earth metals (Group IIA or Group 2), also possess many properties characteristic of metals. They share most of the characteristics of the alkali metals, except that they have slightly higher effective nuclear charges and thus slightly smaller atomic radii. They have two electrons in their valence shell, both of which are easily removed to form divalent cations. Together, the alkali and alkaline earth metals are called the active metals because they are so reactive that they are not naturally found in their elemental (neutral) state.
CHALCOGENS (VIA)
The chalcogens (Group VIA or Group 16) are an eclectic group of nonmetals and metalloids. While not as reactive as the halogens, they are crucial for normal biological functions. They each have six electrons in their valence electron shell and, due to their proximity to the metalloids, generally have small atomic radii and large ionic radii. Oxygen is the most important element in this group for many reasons; it is one of the primary constituents of water, carbohydrates, and other biological molecules. Sulfur is also an important component of certain amino acids and vitamins. Selenium also is an important nutrient for microorganisms and has a role in protection from oxidative stress. The remainder of this group is primarily metallic and generally toxic to living organisms. It is important to note that, at high concentrations, many of these elements—no matter how biologically useful—can be toxic or damaging.
Bridge
Many of the molecules discussed in metabolism, covered in Chapters 9 through 12 of MCAT Biochemistry Review, utilize lighter nontoxic elements from the chalcogen group (oxygen and sulfur). Many of the heavier chalcogens are toxic metals.
HALOGENS (VIIA)
The halogens (Group VIIA or Group 17) are highly reactive nonmetals with seven valence electrons. These elements are desperate to complete their octets by gaining one additional electron. The physical properties of this group are variable. At standard conditions, the halogens range from gaseous (F2 and Cl2) to liquid (Br2) to solid (I2) forms. Their chemical reactivity is more uniform, and, due to their very high electronegativities and electron affinities, they are especially reactive toward the alkali and alkaline earth metals. Fluorine (F) has the highest electronegativity of all the elements. The halogens are so reactive that they are not naturally found in their elemental state but rather as ions (called halides) or diatomic molecules. Diatomic iodine at standard conditions can be seen in Figure 2.12.
 Figure 2.12. Iodine in Standard State (Diatomic Iodine)
Figure 2.12. Iodine in Standard State (Diatomic Iodine)
MCAT Expertise
Halogens are frequently tested on the MCAT. Remember that they only need one more electron to have a noble gas-like electron configuration (full valence shell).
NOBLE GASES (VIIIA)
The noble gases (Group VIIIA or Group 18) are also known as inert gases because they have minimal chemical reactivity due to their filled valence shells. They have high ionization energies, little or no tendency to gain or lose electrons, and (for He, Ne, and Ar, at least), no measurable electronegativities. The noble gases have extremely low boiling points and exist as gases at room temperature. Noble gases have found a commercial niche as lighting sources, as seen in Figure 2.13, due to their lack of reactivity.
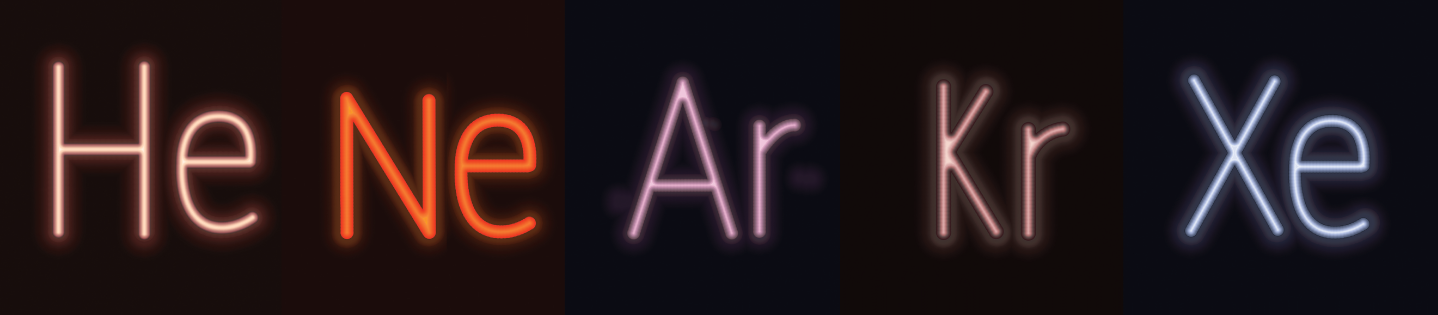 Figure 2.13. Noble Gases Used in “Neon” Signs
Figure 2.13. Noble Gases Used in “Neon” Signs
TRANSITION METALS (B)
The transition elements (Groups IB to VIIIB or Groups 3 to 12) are considered to be metals and as such have low electron affinities, low ionization energies, and low electronegativities. These metals are very hard and have high melting and boiling points. They tend to be quite malleable and are good conductors due to the loosely held electrons that progressively fill the d-orbitals in their valence shells. One of the unique properties of the transition metals is that many of them can have different possible charged forms or oxidation states because they are capable of losing different numbers of electrons from the s- and d-orbitals in their valence shells. For instance, copper (Cu) can exist in either the +1 or the +2 oxidation state, and manganese (Mn) can exist in the +2, +3, +4, +6, or +7 oxidation state. Because of this ability to attain different positive oxidation states, transition metals form many different ionic compounds. These different oxidation states often correspond to different colors; solutions with transition metal-containing complexes are often vibrant, as shown in Figure 2.14.
 Figure 2.14. Solutions of Transition Metal-Containing CompoundsFrom left to right: cobalt(II) nitrate, Co(NO3)2 (red); potassium dichromate, K2Cr2O7 (orange); potassium chromate, K2CrO4 (yellow); nickel(II) chloride, NiCl2 (green); copper(II) sulfate, CuSO4 (blue); potassium permanganate, KMnO4 (violet)
Figure 2.14. Solutions of Transition Metal-Containing CompoundsFrom left to right: cobalt(II) nitrate, Co(NO3)2 (red); potassium dichromate, K2Cr2O7 (orange); potassium chromate, K2CrO4 (yellow); nickel(II) chloride, NiCl2 (green); copper(II) sulfate, CuSO4 (blue); potassium permanganate, KMnO4 (violet)
These complex ions tend to associate in solution either with molecules of water (hydration complexes, such as CuSO4 · 5 H2O) or with nonmetals, (such as [Co(NH3)6]Cl). This ability to form complexes contributes to the variable solubility of certain transition metal-containing compounds. For example, AgCl is insoluble in water but quite soluble in aqueous ammonia due to the formation of the complex ion [Ag(NH3)2]+. The formation of complexes causes the d-orbitals to split into two energy sublevels. This enables many of the complexes to absorb certain frequencies of light—those containing the precise amount of energy required to raise electrons from the lower- to the higher-energy d-orbitals. The frequencies not absorbed (known as the subtraction frequencies) give the complexes their characteristic colors.
Bridge
Many transition metals act as cofactors for enzymes, including vanadium, chromium, manganese, iron, cobalt, nickel, copper, and zinc. Cofactors and coenzymes are discussed in Chapter 2 of MCAT Biochemistry Review.
This brings up an important point about the perception of color: when we perceive an object as a particular color, it because that color is not absorbed—but rather reflected—by the object. If an object absorbs a given color of light and reflects all others, our brain mixes these subtraction frequencies and we perceive the complementary color of the frequency that was absorbed. This is best illustrated with an example. Carotene is a photosynthetic pigment that strongly absorbs blue light but reflects other colors. Thus, our brains interpret the color of carotene as the result of white light minus blue light, which is yellow light. The complementary colors are shown in Figure 2.15; while the MCAT is unlikely to ask you to name the complement of a given color, the relationship between complementary colors, as explained here, is fair game. It should also be noted that the manner in which colors mix in this scheme is distinctly different from mixing, say, paint colors. The differences between these two schemes, termed additive and subtractive color mixing, respectively, are outside the scope of the MCAT.
![]() Figure 2.15. Red—Green—Blue (Additive) Color WheelEach color is directly across the circle from its complementary color; commonly referenced complementary pairs include red/cyan, green/magenta, and blue/yellow.
Figure 2.15. Red—Green—Blue (Additive) Color WheelEach color is directly across the circle from its complementary color; commonly referenced complementary pairs include red/cyan, green/magenta, and blue/yellow.
MCAT Concept Check 2.4:
Before you move on, assess your understanding of the material with this question.
1. For each of the properties listed below, write down the groups of the periodic table that exhibit those properties.
o High reactivity to water:
o Six valence electrons:
o Contain at least one metal:
o Multiple oxidation states:
o Negative oxidation states:
o Possess a full octet in the neutral state: