March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 17. Eliminations
17.B. Regiochemistry of the Double Bond
With some substrates, a β hydrogen is present on only one carbon and (barring rearrangements) there is no doubt as to the identity of the product. For example, PhCH2CH2Br can give only PhCH=CH2. However, in many other cases two or three alkenyl products are possible. In the simplest such case, a sec-butyl compound can give either 1- or 2-butene. There are a number of rules that enable a prediction, in many instances, of which product will predominantly form.82
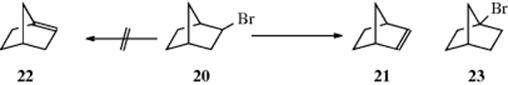
1. No matter the mechanism, a double bond does not go to a bridgehead carbon unless the ring sizes are large enough (Bredt's rule, see Sec. 4.P.iii). This means, for example, not only that 20 gives only 21 and not 22 (indeed 22is not a known compound), but also that 23 does not undergo elimination.
2. No matter the mechanism, if there is a double bond (C=C or C=O) or an aromatic ring already in the molecule that can be in conjugation with the new double bond, the conjugated product usually predominates, sometimes even when the stereochemistry is unfavorable (for an exception, see Sec. 17.C).
3. In the E1 mechanism, the leaving group is gone before the choice is made as to which direction the new double bond takes. Therefore the direction is determined almost entirely by the relative stabilities of the two (or three) possible alkenes. In such cases, Zaitsev's rule83 operates. This rule states that the double bond goes mainly toward the most highly substituted carbon. That is, 3-bromo-2,3-dimethylpentane gives more 2,3-dimethyl-2-pentene than either 3,4-dimethyl-2-pentene or 2-ethyl-3-methyl-1-butene. Thus Zaitsev's rule predicts that the alkene predominantly formed will be the one with the largest possible number of alkyl groups on the C=C carbons, and in most cases this is what is found. From heat of combustion data (see Sec. 1.L), it is known that alkene stability increases with alkyl substitution, although just why this should be is a matter of conjecture. The most common explanation is hyperconjugation. For E1 eliminations, Zaitsev's rule governs the orientation whether the leaving group is neutral or positive, since, as already mentioned, the leaving group is not present when the choice of direction is made. This statement does not hold for E2 eliminations, and it may be mentioned here, for contrast with later results, that E1 elimination of Me2CHCHMeSMe2+ gave 91% of the Zaitsev product and 9% of the other.84 However, there are cases in which the leaving group affects the direction of the double bond in E1 eliminations.85 This may be attributed to ion pairs; that is, the leaving group is not completely gone when the hydrogen departs. Zaitsev's rule breaks down in cases where the non-Zaitsev product is more stable for steric reasons. For example, E1 or E1-like eliminations of 1,2-diphenyl-2-X-propanes (PhMeCXCH2Ph) were reported to give ~ 50% CH2=CPhCH2Ph, despite the fact that the double bond of the Zaitsev product (PhMeC=CHPh) is conjugated with two benzene rings.86
4. For the anti E2 mechanism, a trans β proton is necessary; if this is available in only one direction, that is the way the double bond will form. Because of the free rotation in acyclic systems (except where steric hindrance is great), this is a factor only in cyclic systems. Where trans β hydrogen atoms are available on two or three carbons, two types of behavior are found, depending on substrate structure and the nature of the leaving group. Some compounds follow Zaitsev's rule and give predominant formation of the most highly substituted alkene, but others follow Hofmann's rule: The double bond goes mainly toward the least highly substituted carbon. Although many exceptions are known, the following general statements can be made: In most cases, compounds containing uncharged nucleofuges (those that come off as negative ions) follow Zaitsev's rule, just as they do in E1 elimination, no matter what the structure of the substrate. However, elimination from compounds with charged nucleofuges (e.g., NR3+, SR2+, those that come off as neutral molecules), follow Hofmann's rule if the substrate is acyclic,87 but Zaitsev's rule if the leaving group is attached to a six-membered ring.88
Much work has been devoted to searching for reasons for the differences in orientation. Since Zaitsev orientation almost always gives the thermodynamically more stable isomer, are must explain why in some cases the less stable Hofmann product predominates. Three explanations have been offered for the change in orientation in acyclic systems with a change from uncharged to charged nucleofuges. The first of these89 is that Hofmann orientation is caused by the fact that the acidity of the β hydrogen is decreased by the presence of the electron-donating alkyl groups. For example, under E2 conditions Me2CHCHMeSMe2+ gives more of the
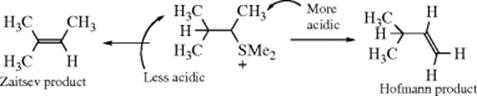
Hofmann product; it is the more acidic hydrogen that is removed by the base. Of course, the CH3 hydrogen atoms would still be more acidic than the Me2CH hydrogen even if a neutral leaving group were present, but the explanation presented was that acidity matters with charged and not with neutral leaving groups, because the charged groups exert a strong electron-withdrawing effect, making differences in acidity greater than they are with the less electron-withdrawing neutral groups.85,90 According to this, the change to a positive leaving group causes the mechanism to shift toward the E1cB end of the spectrum, where there is more C–H bond breaking in the rate-determining step and where, consequently, acidity is more important. In this view, when there is a neutral leaving group, the mechanism is more E1-like, C–X bond breaking is more important, and alkene stability determines the direction of the new double bond.
The third explanation is completely different: Field effects are unimportant, and the difference in orientation is largely a steric effect caused by the fact that charged groups are usually larger than neutral ones. A CH3 group is more open to attack than a CH2R group and a CHR2 group is still less easily attacked. Of course, these considerations also apply when the leaving group is neutral, but they are proposed to be much less important here because the neutral groups are smaller and do not block access to the hydrogen atoms as much. Experiments showed that Hofmann elimination increases with the size of the leaving group. Thus the percentage of 1-ene obtained from CH3CH2CH2CHXCH3 was as follows (X listed in order of increasing size): Br, 31%; I, 30%; OTs, 48%; SMe2+, 87%; SO2Me, 89%; NMe3+, 98%.91 Hofmann elimination was also shown to increase with increase in bulk of the substrate.92 With large enough compounds, Hofmann orientation can be obtained even with halides. tert-Amyl bromide gave 89% of the Hofmann product, for example. Even those who believe in the acidity explanations concede that these steric factors operate in extreme cases.93
There is one series of results incompatible with the steric explanation that E2 elimination from the four 2-halopentanes gave the following percentages of 1-pentene: F, 83%; Cl, 37%; Br, 25%; I, 20%.94 The same order was found for the four 2-halohexanes.95 Although there is some doubt about the relative steric requirements of Br, Cl, and I, there is no doubt that F is the smallest of the halogens, and if the steric explanation were the only valid one, the fluoroalkanes could not give predominant Hofmann orientation. Another result that argues against the steric explanation is the effect of changing the nature of the base. An experiment in which the effective size of the base was kept constant while its basicity was increased (by using as bases a series of XC6H4O− ions) showed that the percentage of Hofmann elimination increased with increasing base strength, although the size of the base did not change.96 These results are in accord with the previous explanation, since an increase in base strength moves an E2 reaction closer to the E1cB end of the spectrum. In further experiments, a large series of bases of different kinds was shown to obey linear free energy relationships between basicity and percentage of Hofmann elimination.97 Certain very large bases (e.g., 2,6-di-tert-butyl-phenoxide) did not obey the relationships and steric effects are important in these cases. How large the base must be before steric effects are observed depends on the pattern of alkyl substitution in the substrate, but not on the nucleofuge.98 One further result may be noted. In the gas phase, elimination of H and BrH+ or H and ClH+ using Me3N as the base predominantly followed Hofmann's rule,99 although BrH+ and ClH+ are not very large.
5. Only a few investigations on the orientation of syn E2 eliminations have been carried out, but these show that Hofmann orientation is greatly favored over Zaitsev.100
6. In the E1cB mechanism, the question of orientation seldom arises because the mechanism is generally found only where there is an electron-withdrawing group in the β position, and that is where the double bond goes.
7. As already mentioned, E2C reactions show a strong preference for Zaitsev orientation.101 In some cases, this can be put to preparative use. For example, the compound PhCH2CHOTsCHMe2 gave ~ 98% PhCH=CHCHMe2under the usual E2 reaction conditions (t-BuOK in t-BuOH). In this case, the double bond goes to the side with more hydrogen atoms because on that side it will be able to conjugate with the benzene ring. However, with the weak base Bu4N+ Br− in acetone the Zaitsev product (PhCH2CH=CMe2) was formed in 90% yield.102