March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 2. Delocalized Chemical Bonding
2.D. Cross-Conjugation51
In a cross-conjugated compound, three groups are present, two of which are not conjugated with each other, although each is conjugated with the third. Some examples are benzophenone (21), triene (22),52 and divinyl ether (23). The MO method shows that the overlap of six p orbitals in 22 (a member of a family of compounds known as dendralenes)52 gives six molecular orbitals, and the three bonding orbitals are shown in Fig. 2.6, along with their energies. Note that two of the carbon atoms do not participate in the α + β orbital.
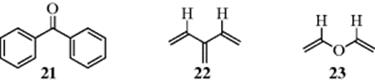
Fig. 2.6 The three bonding orbitals of the dendralene, 3-methylene-1,4-pentadiene (22).
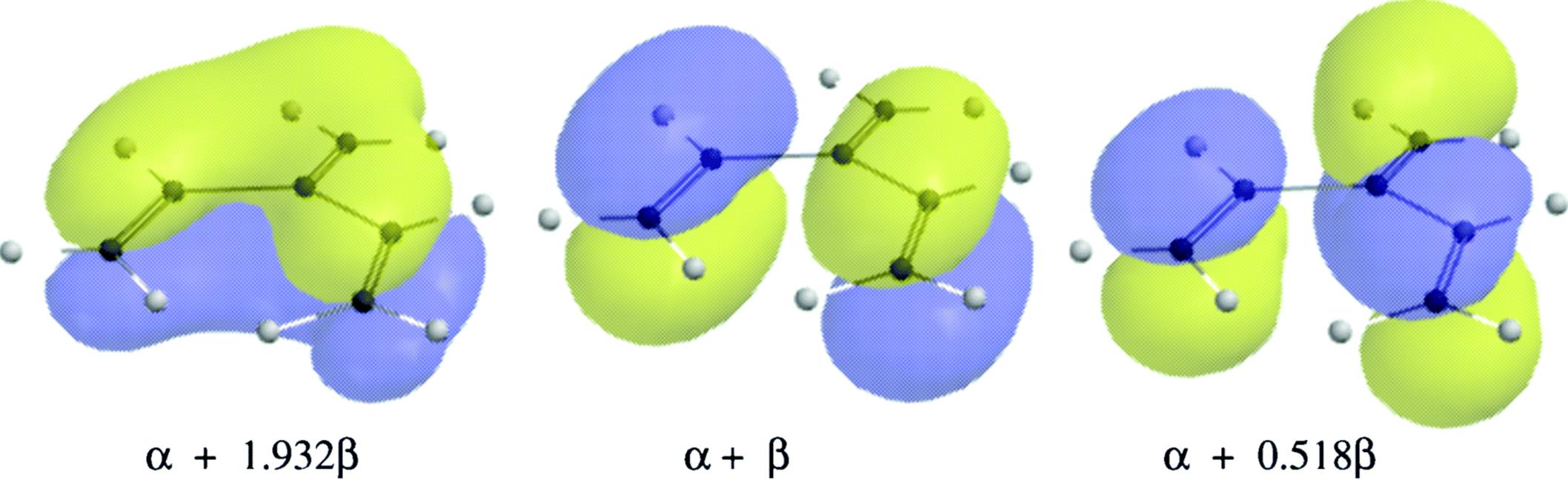
The total energy of the three occupied orbitals is 6α + 6.900β, so the resonance energy is 0.900β. Molecular orbital bond orders are 1.930 for the C-1–C-2 bond, 1.859 for the C-3–C-6 bond, and 1.363 for the C-2–C-3 bond.51Comparing these values with those for butadiene (Sec. 2.C), the C-1–C-2 bond contains more and the C-3–C-6 bond less double-bond character than the double bonds in butadiene. The resonance picture supports this conclusion, since each C-1–C-2 bond is double in three of the five canonical forms, while the C-3–C-6 bond is double in only one. In most cases, it is easier to treat cross-conjugated molecules by the MO method than by the valence bond method.
One consequence of this phenomenon is that the bond length of a cross-conjugated C=C unit is slightly longer than that of a non-cross-conjugated bond. In 24, for example, the cross–conjugated bond is ~ 0.01 Å longer.53 The conjugative effect of a C=C or C![]() C unit can be measured for a conjugated enone: 4.2 kcal mol−1 (17.6 kj mol−1) for an ethenyl substituent, but ~ 2.3 kcal mol−1 (9.6 kj mol−1) for an ethynyl substituent, which is more variable.54
C unit can be measured for a conjugated enone: 4.2 kcal mol−1 (17.6 kj mol−1) for an ethenyl substituent, but ~ 2.3 kcal mol−1 (9.6 kj mol−1) for an ethynyl substituent, which is more variable.54
The phenomenon of homoconjugation is related to cross-conjugation in that there are C=C units in close proximity, but not conjugated one to the other. Homoconjugation arises when the termini of two orthogonal π-systems are held in close proximity, as in compounds with a spiro-tetrahedral carbon atom.55 Spiro[4.4]nonatetraene (25)56 is an example and it is known that the HOMO (see Sec. 15.60) of 25 is raised relative to cyclopentadiene, whereas the LUMO is unaffected.57 Another example is 26, where there are bond length distortions caused by electronic interactions between the unsaturated bicyclic moiety and the cyclopropyl moiety.58 It is assumed that cyclopropyl homoconjugation is responsible for this effect.