March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 2. Delocalized Chemical Bonding
2.E. The Rules of Resonance
One way to express the actual structure of a molecule containing delocalized bonds is to draw several possible structures and to assume that the actual molecule is a hybrid of them. These structures are called canonical forms, but they are not real structures. In other words, the molecule does not rapidly shift between them and a given compound has a single actual structure. That structure is always the same all the time and is taken to be a weighted average of all the canonical forms. Drawing canonical forms and deriving the true structures from them is guided by certain rules, including the following:
1. All the canonical forms must be bona fide Lewis structures (Sec. 1.F). For example, none of them may have a carbon with five bonds.
2. The positions of the nuclei must be the same in all the structures. This means that when drawing the various canonical forms, the electrons are simply arranged in different ways. For this reason, shorthand ways of representing resonance are easy to devise:
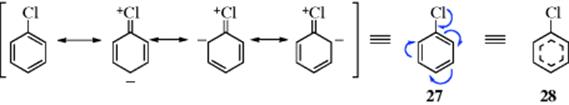
Invoking hyperconjugation (Sec. 2.M), resonance interaction of chlorine with the benzene ring can be represented as shown in 27 or 28 and both representations have been used in the literature to save space. However, the curved-arrow method of 27 will not be used since arrows in this book are used to express the actual movement of electrons in reactions. Representations like 28 will be used occasionally, but more often one or more of the canonical forms will be used. The convention used in dashed-line formulas like 28 is that bonds that are present in all canonical forms are drawn as solid lines, while bonds that are not present in all forms are drawn as dashed lines. A downside of this model is that electron transfer in reactions associated with benzene rings is difficult to track. For this reason, one of the canonical forms is most often used, as mentioned. In most resonance, σ bonds are not involved, and only the π or unshared electrons are utilized. This finding means that writing one canonical form for a molecule, allowed the others to be written by merely moving π and unshared electrons.
3. All atoms taking part in the resonance, (i.e., covered by delocalized electrons), must lie in a plane or nearly so (see Sec. 2.G). This, of course, does not apply to atoms that have the same bonding in all the canonical forms. Maximum overlap of the p orbitals leads to the planarity.
4. All canonical forms must have the same number of unpaired electrons. Thus the diradical structure •CH2CH=CH–CH2• is not a valid canonical form for butadiene.
5. The energy of the actual molecule is lower than that of any form, so delocalization is a stabilizing phenomenon.59
6. All canonical forms do not contribute equally to the true molecule. Each form contributes in proportion to its stability, the most stable form contributing most. Thus, for ethylene, the form +CH2–CH2− has such a high energy compared to CH2=CH2 that it essentially does not contribute at all. This argument was applied to butadiene.39 Equivalent canonical forms, (e.g., 1 and 2) contribute equally. The greater the number of significant structures that can be written and the more nearly equal they are, the greater the resonance energy, other things being equal.
It is not always easy to decide relative stabilities of imaginary structures; the chemist is often guided by intuition.60 However, the following rules may be helpful:
a. Structures with more covalent bonds are ordinarily more stable than those with fewer (cf. 6 and 7).
b. Stability is decreased by an increase in charge separation. Structures with formal charges are less stable than uncharged structures. Structures with more than two formal charges usually contribute very little. An especially unfavorable type of structure is one with two like charges on adjacent atoms.
c. Structures that carry a negative charge on a more electronegative atom are more stable than those in which the charge is on a less electronegative atom. Thus, enolate anion 30 is more stable than canonical form 29. Similarly, positive charges are best carried on atoms of low electronegativity.
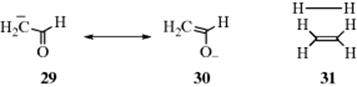
d. Structures with distorted bond angles or lengths are unstable, (e.g., contributor 31 for ethane).