March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 2. Delocalized Chemical Bonding
2.F. The Resonance Effect
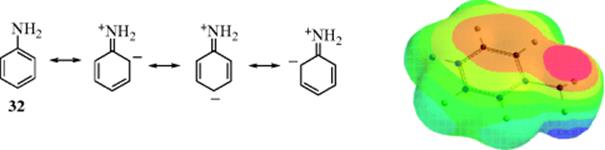
Resonance always results in a different distribution of electron density than would be the case if there were no resonance (i.e., the electrons are dispersed over several atoms rather than concentrated on one atom). For example, if 32were the actual structure of aniline, the two unshared electrons of the nitrogen would reside entirely on that atom. The structure of 32 can be represented as a hybrid that includes contributions from the canonical forms shown, indicating that the electron density of the unshared pair does not reside entirely on the nitrogen, but is spread over the ring. However, as shown by the accompanying electron potential map for aniline, the charge distribution is such that most of the electron density resides on nitrogen. The decrease in electron density at one position (and corresponding increase elsewhere) means that the NH2 contributes or donates electrons to the ring by a resonance effect (“electron releasing,” although no actual contribution takes place), and is called the resonance or mesomeric effect. To emphasize this point, the canonical forms associated with 32 indicate electron release from the nitrogen to the benzene ring (the mesomeric effect), and do not necessarily indicate that there are four canonical forms. In ammonia, where resonance is absent, the unshared pair is located on the nitrogen atom. As with the field effect (Sec. 1.I), a certain molecule (in this case ammonia) maybe thought of as a substrate and effects of substitution on the electron density may be studied. When one of the hydrogen atoms of the ammonia molecule is replaced by a benzene ring (to make aniline, 32), the electrons are “withdrawn” from the ring by the resonance effect, just as when a methyl group replaces a hydrogen atom of benzene, electrons are “donated” by the field effect of the methyl. Note the increassed electron density on the nitrogen in the model, as indicated by the darker area that is above the middle of the model as well and on the nitrogen (on the right side of the model) when compared to benzene in Fig. 2.1b. The idea of donation or withdrawal merely arises from the comparison of a compound with a closely related one or a real compound with a canonical form.