March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 3. Bonding Weaker Than Covalent
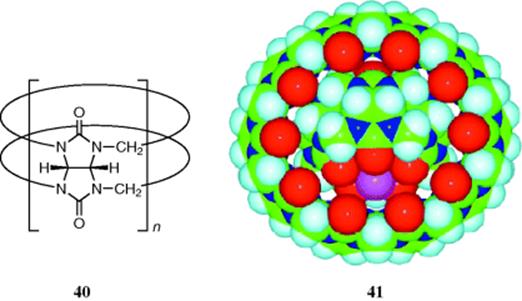
3.E. Cucurbit[n]uril-Based Gyroscane
A new molecule known as gyroscane has been prepared, and proposed as a new supramolecular form.221 The class of compounds known as cucurbit[n]urils, abbreviated Qn (40),222 are condensation products of glycoluril and formaldehyde. These macrocycles can act as molecular hosts. The new “supramolecular form is one in which a smaller macrocycle (Q5) is located inside a larger macrocycle (Q10), with facile rotation of one relative to the other in solution (see 41).221 The image of a ring rotating independently inside another ring, which resembles a gyroscope, suggests the name gyroscane for this new class of supramolecular system.”222
[Reprinted with permission from Day, A.I, Blanch, R.J.; Arnold, A.P.; Lorenzo, S.; Lewis, G.R.; Dance, I. Angew Chem. Int. Ed. 2002, 41, 275, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright © 2002 by Wiley-VCH Verlag.]
Notes
1. For a theoretical treatment, see Becke, A.A.A.; Kannemann, F.O. Can. J. Chem. 2010, 88, 1057.
2. For a discussion of hydrogen bonding in organic synthesis, Hydrogen Bonding in Organic Synthesis, see Pihko, M. (Ed.), Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim, 2009.
3. See Schuster, P.; Zundel, G.; Sandorfy, C. The Hydrogen Bond, 3 Vols., North Holland Publishing Co., Amsterdam, The Netherlands, 1976. For a monograph, see Joesten, M.D.; Schaad, L.J. Hydrogen Bonding, Marcel Dekker, NY, 1974. For reviews, see Meot-Ner, M. Mol. Struct. Energ. 1987, 4, 71; Joesten, M.D. J. Chem. Educ. 1982, 59, 362; Gur'yanova, E.N.; Gol'dshtein, I.P.; Perepelkova, T.I. Russ. Chem. Rev. 1976, 45, 792; Kollman, P.A.; Allen, L.C. Chem. Rev. 1972, 72, 283; Huggins, M.L. Angew. Chem. Int. Ed. 1971, 10, 147; Rochester, C.H. in Patai, S. The Chemistry of the Hydroxyl Group, pt. 1, Wiley, NY, 1971, pp. 327–392. See also, Hamilton, W.C.; Ibers, J.A. Hydrogen Bonding in Solids, W.A. Benjamin, NY, 1968. Also see, Chen, J.; McAllister, M.A.; Lee, J.K.; Houk, K.N. J. Org. Chem. 1998, 63, 4611.
4. See Abraham, M.H.; Platts, J.A. J. Org. Chem. 2001, 66, 3484.
5. Belkova, N.V.; Shubina, E.S.; Epstein, L.M. Acc. Chem. Res. 2005, 38, 624. For a review of hydrogen bonding in cluster ions, see Meot-Ner (Mautner), M. Chem. Rev. 2005, 105, 213.
6. Steiner, T. Angew. Chem. Int. Ed. 2002, 41, 48. See also, Damodharan, L.; Pattabhi, V. Tetrahedron Lett. 2004, 45, 9427.
7. See Nakahara, M.; Wakai, C. Chem. Lett. 1992, 809.
8. Li, C.-J.; Chen, T.-H. Organic Reactions in Aqueous Media, Wiley, NY, 1997.
9. Li, C.-J. Chem. Rev. 1993, 93, 2023.
10. See Curtiss, L.A.; Blander, M. Chem. Rev. 1988, 88, 827.
11. For a review of hydrogen bonding in carboxylic acids and acid derivatives, see Hadž i, D.; Detoni, S. in Patai, S. The Chemistry of Acid Derivatives, pt. 1, Wiley, NY, 1979, pp. 213–266.
12. Emerson, M.T.; Grunwald, E.; Kaplan, M.L.; Kromhout, R.A. J. Am. Chem. Soc. 1960, 82, 6307.
13. For a review of very strong hydrogen bonding, see Emsley, J. Chem. Soc. Rev. 1980, 9, 91.
14. Howard, J.A.K.; Hoy, V.J.; O'Hagan, D.; Smith, G.T. Tetrahedron 1996, 52, 12613. For a discussion of the strength of such hydrogen bonds, see Perrin, C.L. Acc. Chem. Res. 2010, 43, 1550.
15. See Sorensen, J.B.; Lewin, A.H.; Bowen, J.P. J. Org. Chem. 2001, 66, 4105. Also see Ohshima, Y.; Sato, K.; Sumiyoshi, Y.; Endo, Y. J. Am. Chem. Soc. 2005, 127, 1108.
16. Grech, E.; Nowicka-Scheibe, J.; Olejnik, Z.; Lis, T.; Pawêka, Z.; Malarski, Z.; Sobczyk, L. J. Chem. Soc., Perkin Trans. 2 1996, 343. See Steiner, T. J. Chem. Soc., Perkin Trans. 2 1995, 1315.
17. For a comparison of the relative strengths of OH![]() Cl versus OH
Cl versus OH![]() F hydrogen bonds, see Caminati, W.; Melandri, S.; Maris, A.; Paolo Ottaviani, P. Angew. Chem. Int. Ed. 2006, 45, 2438.
F hydrogen bonds, see Caminati, W.; Melandri, S.; Maris, A.; Paolo Ottaviani, P. Angew. Chem. Int. Ed. 2006, 45, 2438.
18. For reviews of the relationship between hydrogen bond strength and acid-base properties, see Pogorelyi, V.K.; Vishnyakova, T.B. Russ. Chem. Rev. 1984, 53, 1154; Epshtein, L.M. Russ. Chem. Rev. 1979, 48, 854.
19. See Abraham, M.H.; Doherty, R.M.; Kamlet, M.J.; Taft, R.W. Chem. Br. 1986, 551; Kamlet, M.J.; Abboud, J.M.; Abraham, M.H.; Taft, R.W. J. Org. Chem. 1983, 48, 2877. For a criticism of the β scale, see Laurence, C.; Nicolet, P.; Helbert, M. J. Chem. Soc., Perkin Trans. 2 1986, 1081. See also, Roussel, C.; Gentric, E.; Sraidi, K.; Lauransan, J.; Guihéneuf, G.; Kamlet, M.J.; Taft, R.W. J. Org. Chem. 1988, 53, 1545; Abraham, M.H.; Grellier, P.L.; Prior, D.V.; Morris, J.J.; Taylor, P.J. J. Chem. Soc., Perkin Trans. 2 1990, 521. Deuterium exchange has been used as an indicator of hydrogen-bond donors and acceptors: see Strobel, T.A.; Hester, K.C.; Sloan Jr., E.D.; Koh, C.A. J. Am. Chem. Soc. 2007, 129, 9544.
20. Kamlet, M.J.; Gal, J.; Maria, P.; Taft, R.W. J. Chem. Soc., Perkin Trans. 2 1985, 1583.
21. Allen, F.H.; Raithby, P.R.; Shields, G.P.; Taylor, R. Chem. Commun. 1998, 1043.
22. Joerg, S.; Drago, R.S.; Adams, J. J. Chem. Soc., Perkin Trans. 2 1997, 2431. See Guerra, C.F.; van der Wijst, T.; Bickelhaupt, F.M. Chem. Eur. J. 2006, 12, 3032; Guerra, C.F.; Zijlstra, H.; Paragi, G.T.; Bickelhaupt, F.M. Chem. Eur. J. 2011, 17, 12612.
23. Stahl, N.; Jencks, W.P. J. Am. Chem. Soc. 1986, 108, 4196.
24. Scheiner, S.; Wang, L. J. Am. Chem. Soc. 1993, 115, 1958.
25. Perrin, C. L. Annu. Rev. Phys. Org. Chem. 1997, 48, 511.
26. Lin, J.; Frey, P. A. J. Am. Chem. Soc. 2000, 122, 11258.
27. Etter, M.C. Acc. Chem. Res. 1990, 23, 120; Taylor, R.; Kennard, O. Acc. Chem. Res. 1984, 17, 320.
28. Stewart, R. The Proton: Applications to Organic Chemistry, Academic Press, NY, 1985, pp. 148–153.
29. A statisical analysis of X-ray crystallographic data has shown that most hydrogen bonds in crystals are nonlinear by ~10–15°: Kroon, J.; Kanters, J.A.; van Duijneveldt-van de Rijdt, J.G.C.M.; van Duijneveldt, F.B.; Vliegenthart, J.A. J. Mol. Struct. 1975, 24, 109. See also, Taylor, R.; Kennard, O.; Versichel, W. J. Am. Chem. Soc. 1983, 105, 5761; 1984, 106, 244.
30. For a discussion of the symmetry of hydrogen bonds in solution, see Perrin, C.L. Pure Appl. Chem. 2009, 81, 571. For reviews of a different aspect of hydrogen bond geometry, see Legon, A.C.; Millen, D.J. Chem. Soc. Rev.1987, 16, 467, Acc. Chem. Res. 1987, 20, 39.
31. Yoshimi, Y.; Maeda, H.; Sugimoto, A.; Mizuno, K. Tetrahedron Lett. 2001, 42, 2341.
32. Emsley, J.; Freeman, N.J.; Parker, R.J.; Dawes, H.M.; Hursthouse, M.B. J. Chem. Soc., Perkin Trans. 1 1986, 471.
33. For some other three-center hydrogen bonds, see Taylor, R.; Kennard, O.; Versichel, W. J. Am. Chem. Soc. 1984, 106, 244; Jeffrey, G.A.; Mitra, J. J. Am. Chem. Soc. 1984, 106, 5546; Staab, H.A.; Elbl, K.; Krieger, C. Tetrahedron Lett. 1986, 27, 5719.
34. Hine, J.; Hahn, S.; Miles, D.E. J. Org. Chem. 1986, 51, 577.
35. Caminati, W.; Fantoni, A.C.; Schäfer, L.; Siam, K.; Van Alsenoy, C. J. Am. Chem. Soc. 1986, 108, 4364.
36. Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond, W.H. Freeman, San Francisco, 1960, p. 260.
37. Chandra, A.K.; Zeegers-Huyskens, T. J. Org. Chem. 2003, 68, 3618.
38. Perrin, C.L.; Kim, Y.-J. J. Am. Chem. Soc. 1998, 120, 12641.
39. Görbitz, C.H.; Etter, M.C. J. Chem. Soc., Perkin Trans. 2 1992, 131.
40. Steiner, T.; Tamm, M.; Lutz, B.; van der Maas, J. Chem. Commun. 1996, 1127.
41. Lakshmi, B.; Samuelson, A.G.; Jovan Jose, K.V.; Gadre, S.R.; Arunan, E. New J. Chem. 2005, 29, 371.
42. See Symons, M.C.R. Chem. Soc. Rev. 1983, 12, 1; Egorochkin, A.N.; Skobeleva, S.E. Russ. Chem. Rev. 1979, 48, 1198; Aaron, H.S. Top. Stereochem. 1979, 11, 1. For a review of the use of rotational spectra to study hydrogen bonding, see Legon, A.C. Chem. Soc. Rev. 1990, 19, 197.
43. Tichy, M. Adv. Org. Chem. 1965, 5, 115 contains a lengthy table of free and intramolecularly hydrogen-bonding peaks. For a discussion of the role of methyl groups in the formation of hydrogen bonds in dimithyl sulfide(DMS)–methanol mixtures, see Li, Q.; Wu, G.; Yu, Z. J. Am. Chem. Soc. 2006, 128, 1438.
44. See Lees, W.A.; Burawoy, A. Tetrahedron 1963, 19, 419.
45. See Davis, Jr., J.C.; Deb, K.K. Adv. Magn. Reson. 1970, 4, 201. Also see, Kumar, G.A.; McAllister, M.A. J. Org. Chem. 1998, 63, 6968.
46. Bruck, A.; McCoy, L.L.; Kilway, K.V. Org. Lett. 2000, 2, 2007. For a discussion of the effect of solvents on hydrogen bonding, see Cook, J.L.; Hunter, C.A.; Low, C.M.R.; Perez-Velasco, A.; Vinter, J.G. Angew. Chem. Int. Ed.2007, 46, 3706.
47. Del Bene, J.E.; Perera, S.A.; Bartlett, R.J. J. Am. Chem. Soc. 2000, 122, 3560.
48. Maiti, N.C.; Zhu, Y.; Carmichael, I.; Serianni, A.S.; Anderson, V.E. J. Org. Chem. 2006, 71, 2878.
49. For reviews of the effect of hydrogen bonding on reactivity, see Hibbert, F.; Emsley, J. Adv. Phys. Org. Chem. 1990, 26, 255; Sadekov, I.D.; Minkin, V.I.; Lutskii, A.E. Russ. Chem. Rev. 1970, 39, 179.
50. For a review, see Pogorelyi, V.K. Russ. Chem. Rev. 1977, 46, 316.
51. See Green, R.D. Hydrogen Bonding by C–H Groups, Wiley, NY, 1974. See also, Nakai, Y.; Inoue, K.; Yamamoto, G.; Öki, M. Bull. Chem. Soc. Jpn. 1989, 62, 2923; Seiler, P.; Dunitz, J.D. Helv. Chim. Acta 1989, 72, 1125.
52. For a theoretical study of weak hydrogen-bonds, see Calhorda, M.J. Chem. Commun. 2000, 801.
53. For a review, see Hopkinson, A.C., in Patai, S. The Chemistry of the Carbon–Carbon Triple Bond, pt. 1, Wiley, NY, 1978, pp. 75–136. See also, DeLaat, A.M.; Ault, B.S. J. Am. Chem. Soc. 1987, 109, 4232.
54. Streiner, T.; Kanters, J.A.; Kroon, J. Chem. Commun. 1996, 1277.
55. See Zuika, I.V.; Bankovskii, Yu.A. Russ. Chem. Rev. 1973, 42, 22; Crampton, M.R. in Patai, S. The Chemistry of the Thiol Group, pt. 1, Wiley, NY, 1974, pp. 379–396; Pogorelyi, V.K. Russ. Chem. Rev. 1977, 46, 316.
56. See Smith, J.W. in Patai, S. The Chemistry of the Carbon–Halogen Bond, pt. 1; Wiley, NY, 1973, pp. 265–300. See also, Bastiansen, O.; Fernholt, L.; Hedberg, K.; Seip, R. J. Am. Chem. Soc. 1985, 107, 7836.
57. Fujimoto, E.; Takeoka, Y.; Kozima, K. Bull. Chem. Soc. Jpn. 1970, 43, 991; Azrak, R.G.; Wilson, E.B. J. Chem. Phys. 1970, 52, 5299.
58. Fujiwara, F.Y.; Martin, J.S. J. Am. Chem. Soc. 1974, 96, 7625; French, M.A.; Ikuta, S.; Kebarle, P. Can. J. Chem. 1982, 60, 1907.
59. In a few cases, the presence of an unsymmetrical cation causes the hydrogen to be closer to one fluorine than to the other: Williams, J.M.; Schneemeyer, L.F. J. Am. Chem. Soc. 1973, 95, 5780.
60. Schaefer, T.; McKinnon, D.M.; Sebastian, R.; Peeling, J.; Penner, G.H.; Veregin, R.P. Can. J. Chem. 1987, 65, 908; Marstokk, K.; Møllendal, H.; Uggerud, E. Acta Chem. Scand. 1989, 43, 26.
61. McDaniel, D.H.; Evans, W.G. Inorg. Chem, 1966, 5, 2180; Sabin, J.R. J. Chem. Phys. 1971, 54, 4675.
62. Calhorda, M.J. Chem. Commun. 2000, 801.
63. Ahlberg, P.; Davidsson, O.; Johnsson, B.; McEwen, I.; Rönnqvist, M. Bull. Soc. Chim. Fr. 1988, 177.
64. Allerhand, A.; Schleyer, P.v.R. J. Am. Chem. Soc. 1963, 85, 866.
65. For example, see Bakke, J.M.; Chadwick, D.J. Acta Chem. Scand. Ser. B 1988, 42, 223: Atwood, J.L.; Hamada, F.; Robinson, K.D.; Orr, G.W.; Vincent, R.L. Nature 1991, 349, 683.
66. Yoshida, Z.; Ishibe, N.; Kusumoto, H. J. Am. Chem. Soc. 1969, 91, 2279.
67. McMurry, J.E.; Lectka, T.; Hodge, C.N. J. Am. Chem. Soc. 1989, 111, 8867. See also, Sorensen, T.S.; Whitworth, S.M. J. Am. Chem. Soc. 1990, 112, 8135.
68. McMurry, J.E.; Lectka, T. Accts. Chem. Res. 1992, 25, 47.
69. Ponec, R.; Yuzhakov, G.; Tantillo, D. J. J. Org. Chem. 2004, 69, 2992.
70. Huggins, M.T.; Lightner, D.A. J. Org. Chem. 2001, 66, 8402.
71. Mazik, M.; Bläser, D.; Boese, R. Tetrahedron 2001, 57, 5791.
72. Cannizzaro, C.E.; Houk, K.N. J. Am. Chem. Soc. 2002, 124, 7163.
73. Cummings, D.L.; Wood, J.L. J. Mol. Struct. 1974, 23, 103.
74. Gallo, E.A.; Gelman, S.H. Tetrahedron Lett. 1992, 33, 7485.
75. Adams, H.; Harris, K.D.M.; Hembury, G.A.; Hunter, C.A.; Livingstone, D.; McCabe, J.F. Chem. Commun. 1996, 2531. See Steiner, T.; Starikov, E.B.; Tamm, M. J. Chem. Soc., Perkin Trans. 2 1996.
76. Allen, F.H.; Howard, J.A.K.; Hoy, V.J.; Desiraju, G.R.; Reddy, D.S.; Wilson, C.C. J. Am. Chem. Soc. 1996, 118, 4081.
77. Tsuzuki, S.; Lüthi, H.P. J. Chem. Phys. 2001, 114, 3949; Arunan, E.; Gutowsky, H S. J. Chem. Phys. 1993, 98, 4294; Felker, P.M.; Maxton, P.M.; Schaeffer, M.W. Chem. Rev. 1994, 94, 1787; Venturo, V.A.; Felker, P.M. J. Chem. Phys. 1993, 99, 748; Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M.; Tanabe, K. J. Am. Chem. Soc. 2002, 124, 104; Hobza, P.; Jure![]() ka, P. J. Am. Chem. Soc. 2003, 125, 15608.
ka, P. J. Am. Chem. Soc. 2003, 125, 15608.
78. Meyer, E.A.; Castellano, R.K.; Diederich, F. Angew. Chem. Int. Ed. 2003, 42, 1210.
79. Sinnokrot, M.O.; Valeev, E.F.; Sherrill, C.D. J. Am. Chem. Soc. 2002, 124, 10887.
80. Sinnokrot, M.O.; Sherrill, C.D. J. Am. Chem. Soc. 2004, 126, 7690.
81. Lindeman, S.V.; Kosynkin, D.; Kochi, J.K. J. Am. Chem. Soc. 1998, 120, 13268; Ma, J.C.; Dougherty, D.A. Chem. Rev. 1997, 97, 1303; Dougherty, D.A. Science 1996, 271, 163; Cubero, E.; Luque, F.J.; Orozco, M. Proc. Natl. Acad. Sci. USA 1998, 95, 5976.
82. Ribas, J.; Cubero, E.; Luque, F.J.; Orozco, M. J. Org. Chem. 2002, 67, 7057.
83. Petersen, S.B.; Led, J.J.; Johnston, E.R.; Grant, D.M. J. Am. Chem. Soc. 1982, 104, 5007.
84. Wakita, M.; Kuroda, Y.; Fujiwara, Y.; Nakagawa, T. Chem. Phys. Lipids 1992, 62, 45.
85. Viel, S.; Mannina, L.; Segre, A. Tetrahedron Lett. 2002, 43, 2515. See also, Ribas, J.; Cubero, E.; Luque, F.J.; Orozco, M. J. Org. Chem. 2002, 67, 7057. For a discussion of substituent effects on aromatic stacking interactions, see Cockroft, S.L.; Perkins, J.; Zonta, C.; Adams, H.; Spey, S.E.; Low, C.M.R.; Vinter, J.G.; Lawson, K.R.; Urch, C.J.; Hunter, C.A. Org. Biomol. Chem.2007, 5, 1062.
86. Foster, R. Organic Charge-Transfer Complexes, Academic Press, NY, 1969; Mulliken, R.S.; Person, W.B. Molecular Complexes, Wiley, NY, 1969; Rose, J. Molecular Complexes, Pergamon, Elmsford, NY, 1967; Poleshchuk, O.Kh.; Maksyutin, Yu.K. Russ. Chem. Rev. 1976, 45, 1077; Banthorpe, D.V. Chem. Rev. 1970, 70, 295; Kosower, E.M. Prog. Phys. Org. Chem. 1965, 3, 81; Foster, R. Chem. Br. 1976, 12, 18.
87. These have often been called charge-transfer complexes, but this term implies that the bonding involves charge transfer, which is not always the case, so that the more neutral name EDA complex is preferable. See Mulliken, R.S.; Person, W.B. J. Am. Chem. Soc. 1969, 91, 3409.
88. Also see Bentley, M.D.; Dewar, M.J.S. Tetrahedron Lett. 1967, 5043.
89. See Collman, J.P.; Hegedus, L.S.; Norton, J.R.; Finke, R.G. Principles and Applications of Organotransition Metal Chemistry, 2nd ed, University Science Books, Mill Valley, CA, 1987; Alper, H. Transition Metal Organometallics in Organic Synthesis, 2 Vols., Academic Press, NY, 1976, 1978. For general reviews, see Churchill, M.R.; Mason, R. Adv. Organomet. Chem. 1967, 5, 93; Cais, M. in Patai, S. The Chemistry of Alkenes, Vol. 1, Wiley, NY, 1964, pp. 335–385; Nakamura, A. J. Organomet. Chem. 1990, 400, 35; Bennett, M.A.; Schwemlein, H.P. Angew. Chem. Int. Ed. 1989, 28, 1296; metals-pentadienyl ions, Powell, P. Adv. Organomet. Chem. 1986, 26, 125; complexes of main group metals. For a list of review articles on this subject, see Bruce, M.I. Adv. Organomet. Chem. 1972, 10, 273, pp. 317–321.
90. For a discussion of how this system originated, see Cotton, F.A. J. Organomet. Chem. 1975, 100, 29.
91. Another prefix used for complexes is μ (mu), which indicates that the ligand bridges two metal atoms.
92. See Pearson, A.J. Metallo-organic Chemistry Wiley, NY, 1985; Ittel, S.D.; Ibers, J.A. Adv. Organomet. Chem. 1976, 14, 33; Hartley, F.R. Chem. Rev. 1973, 73, 163; Angew. Chem. Int. Ed. 1972, 11, 596.
93. Dewar, M.J.S. Bull. Soc. Chim. Fr. 1951, 18, C79.
94. Hu, J.; Gokel, G.W.; Barbour, L.J. Chem. Commun. 2001, 1858.
95. See Zeiss, H.; Wheatley, P.J.; Winkler, H.J.S. BenzenoidMetal Complexes, Ronald Press, NY, 1966.
96. Nicholls, B.; Whiting, M.C. J. Chem. Soc. 1959, 551. For reviews of arene–transition metal complexes, see Uemura, M. Adv. Met.-Org. Chem. 1991, 2, 195; Silverthorn, W.E. Adv. Organomet. Chem. 1975, 13, 47.
97. Landesberg, J.M.; Sieczkowski, J. J. Am. Chem. Soc. 1971, 93, 972.
98. See Parini, V.P. Russ. Chem. Rev. 1962, 31, 408; for a review of complexes in which the acceptor is an organic cation, see Kampar, V.E. Russ. Chem. Rev. 1982, 51, 107; also see, Ref. 86.
99. For a review of quinone complexes, see Foster, R.; Foreman, M.I. in Patai, S. The Chemistry of the Quinonoid Compounds, pt. 1, Wiley, NY, 1974, pp. 257–333.
100. See Blackstock, S.C.; Lorand, J.P.; Kochi, J.K. J. Org. Chem. 1987, 52, 1451.
101. See Foster, R. in Patai, S. The Chemistry of Acid Derivatives, pt. 1, Wiley, NY, 1979, pp. 175–212.
102. See Melby, L.R. in Rappoport, Z. The Chemistry of the Cyano Group, Wiley, NY, 1970, pp. 639–669. See also, Fatiadi, A.J. Synthesis 1987, 959.
103. For reviews, see Bender, C.J. Chem. Soc. Rev. 1986, 15, 475; Kampar, E.; Neilands, O. Russ. Chem. Rev. 1986, 55, 334; Bent, H.A. Chem. Rev. 1968, 68, 587.
104. See, for example, Le Fevre, R.J.W.; Radford, D.V.; Stiles, P.J. J. Chem. Soc. B 1968, 1297.
105. Mulliken, R.S.; Person, W.B. J. Am. Chem. Soc. 1969, 91, 3409.
106. See Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, 3 Vols., Academic Press, NY, 1984; Vögtle, F. Host Guest Complex Chemistry I, II, and III (Top. Curr. Chem.1998, 101, 121), Springer, Berlin, 1981, 1982, 1984; Vögtle, F.; Weber, E. Host Guest Complex Chemistry/Macrocycles, Springer, Berlin, 1985; Izatt, R.M.; Christensen, J.J. Synthetic Multidentate Macrocyclic Compounds, Academic Press, NY, 1978. For reviews, see McDaniel, C.W.; Bradshaw, J.S.; Izatt, R.M. Heterocycles, 1990, 30, 665; Sutherland, I.O. Chem. Soc. Rev. 1986, 15, 63; Franke, J.; Vögtle, F. Top. Curr. Chem. 1986, 132, 135; Cram, D.J. Angew. Chem. Int. Ed. 1986, 25, 1039; Gutsche, C.D. Acc. Chem. Res. 1983, 16, 161; Tabushi, I.; Yamamura, K. Top. Curr. Chem. 1983, 113, 145; Stoddart, J.F. Prog. Macrocyclic Chem. 1981, 2, 173; Cram, D.J.; Cram, J.M. Acc. Chem. Res. 1978, 11, 8; Science, 1974, 183, 803; Gokel, G.W.; Durst, H.D. Synthesis 1976, 168; Aldrichim. Acta 1976, 9, 3; Lehn, J.M. Struct. Bonding (Berlin) 1973, 16, 1; Christensen, J.J.; Eatough, D.J.; Izatt, R.M. Chem. Rev. 1974, 74, 351; Pedersen, C.J.; Frensdorff, H.K. Angew. Chem. Int. Ed. 1972, 11, 16. For reviews of acyclic molecules with similar properties, see Vögtle, E. Chimia 1979, 33, 239; Vögtle, E.; Weber, E. Angew. Chem. Int. Ed. 1979, 18, 753. See Angew. Chem. Int. Ed. 1988, 27, pp. 1021, 1009, 89; and Chem. Scr., 1988, 28, pp. 229, 263, 237. See also, the series Advances in Supramolecular Chemistry.
107. Cook, F.L.; Caruso, T.C.; Byrne, M.P.; Bowers, C.W.; Speck, D.H.; Liotta, C. Tetrahedron Lett. 1974, 4029.
108. Discovered by Pedersen, C.J. J. Am. Chem. Soc. 1967, 89, 2495, 7017. For an account of the discovery, see Schroeder, H.E.; Petersen, C.J. Pure Appl. Chem. 1988, 60, 445.
109. See Inoue, Y.; Gokel, G.W. Cation Binding by Macrocycles, Marcel Dekker, NY, 1990.
110. See Izatt, R.M.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.J.; Sen, D. Chem. Rev. 1985, 85, 271; Parsonage, N.G.; Staveley, L.A.K. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 3, Academic Press, NY, 1984, pp. 1–36.
111. Anet, F.A.L.; Krane, J.; Dale, J.; Daasvatn, K.; Kristiansen, P.O. Acta Chem. Scand. 1973, 27, 3395.
112. See Dale, J.; Eggestad, J.; Fredriksen, S.B.; Groth, P. J. Chem. Soc., Chem. Commun. 1987, 1391; Dale, J.; Fredriksen, S.B. Pure Appl. Chem. 1989, 61, 1587.
113. Izatt, R.M.; Nelson, D.P.; Rytting, J.H.; Haymore, B.L.; Christensen, J.J. J. Am. Chem. Soc. 1971, 93, 1619.
114. Kimura, Y.; Iwashima, K.; Ishimori, T.; Hamaguchi, H. Chem. Lett. 1977, 563.
115. Raevsky, O.A.; Solov'ev, V.P.; Solotnov, A.F.; Schneider, H.-J.; Rüdiger, V. J. Org. Chem. 1996, 61, 8113.
116. Crown ethers have been used to separate isotopes of cations, (e.g., 44Ca from 40Ca). For a review, see Heumann, K.G. Top. Curr. Chem. 1985, 127, 77.
117. For reviews, see Vögtle, F.; Müller, W.M.; Watson, W.H. Top. Curr. Chem. 1984, 125, 131; Weber, E. Prog. Macrocycl. Chem. 1987, 3, 337; Diederich, F. Angew. Chem. Int. Ed. 1988, 27, 362.
118. See van Staveren, C.J.; van Eerden, J.; van Veggel, F.C.J.M.; Harkema, S.; Reinhoudt, D.N. J. Am. Chem. Soc. 1988, 110, 4994. See also, Rodrigue, A.; Bovenkamp, J.W.; Murchie, M.P.; Buchanan, G.W.; Fortier, S. Can. J. Chem. 1987, 65, 2551; Fraser, M.E.; Fortier, S.; Markiewicz, M.K.; Rodrigue, A.; Bovenkamp, J.W. Can. J. Chem. 1987, 65, 2558.
119. Voronkov, M.G.; Knutov, V.I. Sulfur Rep. 1986, 6, 137, Russ. Chem. Rev. 1982, 51, 856; Reid, G.; Schröder, M. Chem. Soc. Rev. 1990, 19, 239.
120. For a review of 17 and its derivatives, see Chaudhuri, P.; Wieghardt, K. Prog. Inorg. Chem. 1987, 35, 329. N-Aryl-azacrown ethers are known, see Zhang, X.-X.; Buchwald, S.L. J. Org. Chem. 2000, 65, 8027.
121. Gersch, B.; Lehn, J.-M.; Grell, E. Tetrahedron Lett. 1996, 37, 2213.
122. Newcomb, M.; Gokel, G.W.; Cram, D.J. J. Am. Chem. Soc. 1974, 96, 6810.
123. Ragunathan, K.G.; Shukla, R.; Mishra, S.; Bharadwaj, P.K. Tetrahedron Lett. 1993, 34, 5631.
124. See Potvin, P.G.; Lehn, J.M. Prog. Macrocycl. Chem. 1987, 3, 167; Kiggen, W.; Vögtle, F. Prog. Macrocycl. Chem. 1987, 3, 309; Dietrich, B. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 2, Academic Press, NY, 1984, pp. 337–405; Parker, D. Adv. Inorg. Radichem. 1983, 27, 1; Lehn, J.M. Acc. Chem. Res. 1978, 11, 49, Pure Appl. Chem. 1977, 49, 857.
125. Shivanyuk, A.; Spaniol, T.P.; Rissanen, K.; Kolehmainen, E.; Böhmer, V. Angew. Chem. Int. Ed. 2000, 39, 3497.
126. Bryany, J.A.; Ho, S.P.; Knobler, C.B.; Cram, D.J. J. Am. Chem. Soc. 1990, 112, 5837.
127. Shinkai, S. Tetrahedron 1993, 49, 8933.
128. See Vicens, J.; Böhmer, V. Calixarenes: A Versatile Class of Macrocyclic Compounds, Kluver: Dordrecht, 1991; Gutsche, C.D. Calixarenes; Royal Society of Chemistry, Cambridge, 1989; Gutsche, C.D. Prog. Macrocycl. Chem. 1987, 3, 93. Also see, Geraci, C.; Piattelli, M.; Neri, P. Tetrahedron Lett. 1995, 36, 5429; Zhong, Z.-L.; Chen, Y.-Y.; Lu, X.-R. Tetrahedron Lett. 1995, 36, 6735; No, K.; Kim, J.E.; Kwon, K.M. Tetrahedron Lett. 1995,36, 8453.
129. Agbaria, K.; Aleksiuk, O.; Biali, S.E.; Böhmer, V.; Frings, M.; Thondorf, I. J. Org. Chem. 2001, 66, 2891. See Agbaria, K.; Biali, S.E.; Böhmer, V.; Brenn, J.; Cohen, S.; Frings, M.; Grynszpan, F.; Harrowfield, J.Mc B.; Sobolev, A.N.; Thondorf, I. J. Org. Chem. 2001, 66, 2900.
130. Cerioni, G.; Biali, S.E.; Rappoport, Z. Tetrahedron Lett. 1996, 37, 5797; Molard, Y.; Bureau, C.; Parrot-Lopez, H.; Lamartine, R.; Regnourf-de-Vains, J.-B. Tetrahedron Lett. 1999, 40, 6383.
131. Otsuka, H.; Araki, K.; Matsumoto, H.; Harada, T.; Shinkai, S. J. Org. Chem. 1995, 60, 4862.
132. Kanamathareddy, S.; Gutsche, C.D. J. Org. Chem. 1994, 59, 3871.
133. Cunsolo, F.; Consoli, G.M.L.; Piattelli, M.; Neri, P. Tetrahedron Lett. 1996, 37, 715.
134. Miyazaki, Y.; Kanbara, T.; Yamamoto, T. Tetrahedron Lett. 2002, 43, 7945; Khan, I.U.; Takemura, H.; Suenaga, M.; Shinmyozu, T.; Inazu, T. J. Org. Chem. 1993, 58, 3158.
135. Masci, B. J. Org. Chem. 2001, 66, 1497; Seri, N.; Thondorf, I.; Biali, S.E. J. Org. Chem. 2004, 69, 4774; Tsubaki, K.; Morimoto, T.; Otsubo, T.; Kinoshita, T.; Fuji, K. J. Org. Chem. 2001, 66, 4083.
136. Stewart, D.R.; Gutsche, C.D. J. Am. Chem. Soc. 1999, 121, 4136.
137. Mascal, M.; Naven, R.T.; Warmuth, R. Tetrahedron Lett. 1995, 36, 9361.
138. Wu, Y.; Shen, X.-P.; Duan, C.-y.; Liu, Y.-i.; Xu, Z. Tetrahedron Lett. 1999, 40, 5749.
139. Colby, D.A.; Lash, T.D. J. Org. Chem.2002, 67, 1031.
140. Akine, S.; Goto, K.; Kawashima, T. Tetrahedron Lett. 2000, 41, 897.
141. Aeungmaitrepirom, W.; Hagège, A.; Asfari, Z.; Bennouna, L.; Vicens, J.; Leroy, M. Tetrahedron Lett. 1999, 40, 6389.
142. Shirakawa, S.; Moriyama, A.; Shimizu, S. Eur. J. Org. Chem. 2008, 5957.
143. Shimizu, S.; Shirakawa, S.; Sasaki, Y.; Hirai, C. Angew. Chem. Int. Ed. 2000, 39, 1256.
144. Stephan, H.; Gloe, K.; Paulus, E.F.; Saadioui, M.; Böhmer, V. Org. Lett. 2000, 2, 839; Asfari, Z.; Thuéry, P.; Nierlich, M.; Vicens, J. Tetrahedron Lett. 1999, 40, 499; Geraci, C.; Piattelli, M.; Neri, P. Tetrahedron Lett. 1996, 37, 3899; Pappalardo, S.; Petringa, A.; Parisi, M.F.; Ferguson, G. Tetrahedron Lett. 1996, 37, 3907.
145. Pulpoka, B.; Asfari, Z.; Vicens, J. Tetrahedron Lett. 1996, 37, 6315.
146. Makrlík, E.; Va![]() ura, P. Monat. Chemie 2006, 137, 1185-.
ura, P. Monat. Chemie 2006, 137, 1185-.
147. See Collet, A. Tetrahedron 1987, 43, 5725, in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 1, Academic Press, NY, 1984, pp. 97–121.
148. Lein, G.M.; Cram, D.J. J. Am. Chem. Soc. 1985, 107, 448.
149. Fo Kron, T.E.; Tsvetkov, E.N. Russ. Chem. Rev. 1990, 59, 283; Menger, F.M. Top. Curr. Chem. 1986, 136, 1.
150. Tümmler, B.; Maass, G.; Weber, E.; Wehner, W.; Vögtle, F. J. Am. Chem. Soc. 1977, 99, 4683.
151. Vögtle, F.; Weber, E. Angew. Chem. Int. Ed. 1974, 13, 814.
152. For the synthesis of N-pivot lariat ethers, see Elwahy, A.H.M.; Abbas, A.A. J. Het. Chem. 2008, 45, 1.
153. Gatto, V.J.; Gokel, G.W. J. Am. Chem. Soc. 1984, 106, 8240; Nakatsuji, Y.; Nakamura, T.; Yonetani, M.; Yuya, H.; Okahara, M. J. Am. Chem. Soc. 1988, 110, 531.
154. Lee, W.Y.; Park, C.H. J. Org. Chem. 1993, 58, 7149.
155. Wang, T.; Bradshaw, J.S.; Izatt, R.M. J. Heterocylic Chem. 1994, 31, 1097.
156. Fujimoto, T.; Yanagihara, R.; Koboyashi, K.; Aoyama, Y. Bull. Chem. Soc. Jpn. 1995, 68, 2113.
157. For reviews, see Rebek, Jr., J. Angew. Chem. Int. Ed. 1990, 29, 245; Acc. Chem. Res. 1990, 23, 399; Top. Curr. Chem. 1988, 149, 189; Diederich, F. J. Chem. Educ. 1990, 67, 813; Hamilton, A.D. J. Chem. Educ. 1990, 67, 821; Raevskii, O.A. Russ. Chem. Rev. 1990, 59, 219.
158. Mageswaran, R.; Mageswaran, S.; Sutherland, I.O. J. Chem. Soc., Chem. Commun. 1979, 722.
159. Diederich, F.; Dick, K. J. Am. Chem. Soc. 1984, 106, 8024; Diederich, F.; Griebe, D. J. Am. Chem. Soc. 1984, 106, 8037. See also, Vögtle, F.; Müller, W.M.; Werner, U.; Losensky, H. Angew. Chem. Int. Ed. 1987, 26, 901.
160. Hosseini, M.W.; Lehn, J.M. J. Am. Chem. Soc. 1987, 109, 7047. For a discussion, see Mertes, M.P.; Mertes, K.B. Acc. Chem. Res. 1990, 23, 413.
161. See Cram, D.J. Angew. Chem. Int. Ed. 1986, 25, 1039.
162. See Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vols. 1–3, Academic Press, NY, 1984; Weber, E. Top. Curr. Chem. 1987, 140, 1; Gerdil, R. Top. Curr. Chem. 1987, 140, 71; Mak, T.C.W.; Wong, H.N.C. Top. Curr. Chem. 1987, 140, 141; Bishop, R.; Dance, I.G. Top. Curr. Chem. 1988, 149, 137.
163. For reviews, see Goldberg, I. Top. Curr. Chem. 1988, 149, 1; Weber, E.; Czugler, M. Top. Curr. Chem. 1988, 149, 45; MacNicol, D.D.; McKendrick, J.J.; Wilson, D.R. Chem. Soc. Rev. 1978, 7, 65.
164. Strobel, T.A.; Hester, K.C.; Sloan Jr., E.D.; Koh, C.A. J. Am. Chem. Soc. 2007, 129, 9544.
165. For a review of urea and thiourea inclusion compounds, see Takemoto, K.; Sonoda, N. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 2, Academic Press, NY, 1984, pp. 47–67.
166. Taken from Alavi, S.; Udachin, K.; Ripmeester, J.A. Chem. Eur. J. 2010, 16, 1017.
167. Radell, J.; Connolly, J.W.; Cosgrove, Jr., W.R. J. Org. Chem. 1961, 26, 2960.
168. Redlich, O.; Gable, C.M.; Dunlop, A.K.; Millar, R.W. J. Am. Chem. Soc. 1950, 72, 4153.
169. Heaton, N.J.; Vold, R.L.; Vold, R.R. J. Am. Chem. Soc. 1989, 111, 3211.
170. For a review, see MacNicol, D.D. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 2, Academic Press, NY, 1984, pp. 1–45.
171. Toda, F.; Hyoda, S.; Okada, K.; Hirotsu, K. J. Chem. Soc., Chem. Commun. 1995, 1531.
172. For a monograph on water clathrates, see Berecz, E.; Balla-Achs, M. Gas Hydrates; Elsevier, NY, 1983. For reviews, see Jeffrey, G.A. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 1, Academic Press, NY, 1984, pp. 135–190; Cady, G.H. J. Chem. Educ. 1983, 60, 915.
173. Sloan, E.D. Clathrate Hydrate of Natural Gases, Marcel Dekker, Inc., 1998.
174. Kirkor, E.; Gebicki, J.; Phillips, D.R.; Michl, J. J. Am. Chem. Soc. 1986, 108, 7106.
175. See also, Toda, F. Pure App. Chem. 1990, 62, 417, Top. Curr. Chem. 1988, 149, 211; 1987, 140, 43; Davies, J.E.; Finocchiaro, P.; Herbstein, F.H. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 2, Academic Press, NY, 1984, pp. 407–453.
176. For a review, see Giglio, E. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 2, Academic Press, NY, 1984, pp. 207–229.
177. See Miki, K.; Masui, A.; Kasei, N.; Miyata, M.; Shibakami, M.; Takemoto, K. J. Am. Chem. Soc. 1988, 110, 6594.
178. Barbour, L.J.; Caira, M.R.; Nassimbeni, L.R. J. Chem. Soc., Perkin Trans. 2 1993, 2321. Also see, Barbour, L.J.; Caira, M.R.; Nassimbeni, L.R. J. Chem. Soc., Perkin Trans. 2 1993, 1413.
179. Lämsä, M.; Suorsa, T.; Pursiainen, J.; Huuskonen, J.; Rissanen, K. Chem. Commun. 1996, 1443.
180. Sherman, J.C.; Knobler, C.B.; Cram, D.J. J. Am. Chem. Soc. 1991, 113, 2194.
181. van Wageningen, A.M.A.; Timmerman, P.; van Duynhoven, J.P.M.; Verboom, W.; van Veggel, F.C.J.M.; Reinhoudt, D.N. Chem. Eur.J. 1997, 3, 639; Fraser, J.R.; Borecka, B.; Trotter, J.; Sherman, J.C. J. Org. Chem. 1995, 60, 1207; Place, D.; Brown, J.; Deshayes, K. Tetrahedron Lett. 1998, 39, 5915. See also: Jasat, A.; Sherman, J.C. Chem. Rev. 1999, 99, 931.
182. Chapman, R.G.; Sherman, J.C. J. Org. Chem. 2000, 65, 513.
183. See Bender, M.L.; Komiyama, M. Cyclodextrin Chemistry, Springer, NY, 1978. For reviews, see in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Academic Press, NY, 1984, the reviews, by Saenger, W. Vol. 2, pp. 231–259, Bergeron, R.J. Vol. 3, pp. 391–443, Tabushi, I. Vol. 3, pp. 445–471, Breslow, R. Vol. 3, pp. 473–508; Croft, A.P.; Bartsch, R.A. Tetrahedron 1983, 39, 1417; Tabushi, I.; Kuroda, Y. Adv. Catal., 1983, 32, 417; Tabushi, I. Acc. Chem. Res. 1982, 15, 66; Saenger, W. Angew. Chem. Int. Ed. 1980, 19, 344; Bergeron, R. J. Chem. Ed. 1977, 54, 204; Griffiths, D.W.; Bender, M.L. Adv. Catal. 1973, 23, 209.
184. García-Río, L.; Hall, R.W.; Mejuto, J.C.; Rodriguez-Dafonte, P. Tetrahedron 2007, 63, 2208.
185. Szejtli, J. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 3, Academic Press, NY, 1984, p. 332; Nickon, A.; Silversmith, E.F. The Name Game, Pergamon, Elmsford, NY, p. 235.
186. Modified from Saenger, W.; Beyer, K.; Manor, P.C. Acta Crystallogr. Sect. B, 1976, 32, 120.
187. For reviews, see Pagington, J.S. Chem. Br., 1987, 23, 455; Szejtli, J. in Atwood, J.L.; Davies, J.E.; MacNicol, D.D. Inclusion Compounds, Vol. 3, Academic Press, NY, 1984, pp. 331–390.
188. See Saenger, W. Angew. Chem. Int. Ed. 1980, 19, 344.
189. Engeldinger, E.; Armspach, D.; Matt, D. Chem. Rev. 2003, 103, 4147.
190. For a monograph, see Schill, G. Catenanes, Rotaxanes, and Knots, Academic Press, NY, 1971. For a review, see Schill, G. in Chiurdoglu, G. Conformational Analysis, Academic Press, NY, 1971, pp. 229–239.
191. Solladié, N.; Chambron, J.-C.; Sauvage, J.-P. J. Am. Chem. Soc. 1999, 121, 3684.
192. Sasabe, H.; Kihara, N.; Furusho, Y.; Mizuno, K.; Ogawa, A.; Takata, T. Org. Lett. 2004, 6, 3957.
193. Safarowsky, O.; Vogel, E.; Vögtle, F. Eur. J. Org. Chem. 2000, 499.
194. Amabilino, D.B.; Ashton, P.R.; Balzani, V.; Boyd, S.E.; Credi, A.; Lee, J.Y.; Menzer, S.; Stoddart, J.F.; Venturi, M.; Williams, D.J. J. Am. Chem. Soc. 1998, 120, 4295.
195. Chiu, S.-H.; Rowan, S.J.; Cantrill, S.J.; Ridvan, L.; Ashton, R.P.; Garrell, R.L.; Stoddart, J.-F. Tetrahedron 2002, 58, 807; Roh, S.-G.; Park, K.-M.; Park, G.-J.; Sakamoto, S.; Yamaguchi, K.; Kim, K. Angew. Chem. Int. Ed.1999, 38, 638.
196. See Onagi, H.; Easton, C.J.; Lincoln, S.F. Org. Lett. 2001, 3, 1041; Cantrill, S.J.; Youn, G.J.; Stoddart, J.F.; Williams, D.J. J. Org. Chem. 2001, 66, 6857.
197. Chiu, S.-H.; Pease, A.R.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Angew. Chem. Int. Ed. 2002, 41, 270.
198. Schwierz, H.; Vögtle, F. Synthesis 1999, 295.
199. Elizarov, A.M.; Chiu, S.-H.; Stoddart, J.-F. J. Org. Chem. 2002, 67, 9175.
200. MacLachlan, M. J.; Rose, A.; Swager, T. M. J. Am. Chem. Soc. 2001, 123, 9180.
201. Amabilino, D.B.; Ashton, P.R.; Boyd, S.E.; Gómez-López, M.; Hayes, W.; Stoddart, J.F. J. Org. Chem. 1997, 62, 3062.
202. For discussions, see Schill, G. Catenanes, Rotaxanes, and Knots, Academic Press, NY, 1971. For a review, see Schill, G. in Chiurdoglu, G. Conformational Analysis, Academic Press, NY, 1971, pp. 229–239; Walba, D.M. Tetrahedron 1985, 41, 3161.
203. Fujita, M.; Ibukuro, F.; Seki, H.; Kamo, O.; Imanari, M.; Ogura, K. J. Am. Chem. Soc. 1996, 118, 899.
204. Harrison, I.T.; Harrison, S. J. Am. Chem. Soc. 1967, 89, 5723; Ogino, H. J. Am. Chem. Soc. 1981, 103, 1303; Harrison, I.T. J. Chem. Soc., Perkin Trans. 1 1974, 301; Schill, G.; Beckmann, W.; Schweikert, N.; Fritz, H. Chem. Ber. 1986, 119, 2647. See also, Agam, G.; Graiver, D.; Zilkha, A. J. Am. Chem. Soc. 1976, 98, 5206.
205. For a directed synthesis of a rotaxane, see Schill, G.; Zürcher, C.; Vetter, W. Chem. Ber. 1973, 106, 228.
206. Deleuze, M. S.; Leigh, D. A; Zerbetto, F. J. Am. Chem. Soc. 1999, 121, 2364.
207. For the synthesis of a doubly interlocking [2]catenane, see Ibukuro, F.; Fujita, M.; Yamaguchi, K.; Sauvage, J.-P. J. Am. Chem. Soc. 1999, 121, 11014.
208. See Lukin, O.; Godt, A.; Vögtle, F. Chem. Eur. J., 2004, 10, 1879.
209. Frisch, H.L.; Wasserman, E. J. Am. Chem. Soc. 1961, 83, 3789.
210. Molecular Catenanes, Rotaxanes and Knots (Eds., Sauvage, J.-P.; Dietrich-Buchecker, C.O.) Wiley-VCH, Weinheim, 1999; Ashton, P.R.; Bravo, J.A.; Raymo, F.M.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Eur. J. Org. Chem. 1999, 899; Mitchell, D.K.; Sauvage, J.-P. Angew. Chem. Int. Ed. 1988, 27, 930; Nierengarten, J.-F.; Dietrich-Buchecker, C.O.; Sauvage, J.-P. J. Am. Chem. Soc. 1994, 116, 375; Chen, C.-T.; Gantzel, P.; Siegel, J.S.; Baldridge, K.K.; English, R.B.; Ho, D.M. Angew. Chem. Int. Ed. 1995, 34, 2657.
211. Kaida, T.; Okamoto, Y.; Chambron, J.-C.; Mitchell, D.K.; Sauvage, J.-P. Tetrahedron Lett. 1993, 34, 1019.
212. Schmieder, R.; Hübner, G.; Seel, C.; Vögtle, F. Angew. Chem. Int. Ed. 1999, 38, 3528.
213. Prelog, V.; Gerlach, H. Helv. Chim. Acta 1964, 47, 2288; Gerlach, H.; Owtischinnkow, J.A.; Prelog, V. Helv. Chim. Acta 1964, 47, 2294; Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds, Wiley, NY, 1994, pp. 1176–1181; Chorev, M.; Goodman, M. Acc. Chem. Res. 1993, 26, 266; Mislow, K. Chimia, 1986, 40, 395.
214. For an example, see Anelli, P.L.; Spencer, N.; Stoddart, J.F. J. Am. Chem. Soc. 1991, 113, 5131.
215. Oshikiri, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. J. Am. Chem. Soc. 2005, 127, 12186.
216. Anelli, P.L.; Spencer, N.; Stoddart, J.F. J. Am. Chem. Soc. 1991, 113, 5131. For a review of the synthesis and properties of molecules of this type, see Philp, D.; Stoddart, J.F. Synlett 1991, 445.
217. Diederich, F.; Dietrich-Buchecker, C.O.; Nierengarten, S.-F.; Sauvage, J.-P. J. Chem. Soc., Chem. Commun. 1995, 781.
218. Dietrich-Buchecker, C.O.; Nierengarten, J.-F.; Sauvage, J.-P. Tetrahedron Lett. 1992, 33, 3625. See Dietrich-Buchecker, C.O.; Guilhem, J.; Pascard, C.; Sauvage, J.-P. Angew. Chem. Int. Ed. 1990, 29, 1154.
219. Liu, L.F.; Depew, R.E.; Wang, J.C. J. Mol. Biol. 1976, 106, 439.
220. Williams, A.R.; Northrop, B.N.; Chang, T.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Angew. Chem. Int. Ed. 2006, 45, 6665.
221. Day, A.I.; Blanch, R.J.; Arnold, A.P.; Lorenzo, S.; Lewis, G.R.; Dance, I. Angew. Chem. Int. Ed,. 2002, 41, 275.
222. Mock, W.L. Top. Curr. Chem. 1995, 175, 1; Mock, W.L. in Comprehensive Supramolecular Chemistry, Vol. 2 (Eds.: Atwood, J.L.; Davies, J.E.D.; MacNicol, D.D.; Vogtle, F.), Pergamon, Oxford, 1996, pp. 477–493; Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. J. Org. Chem. 2001, 66, 8094. For cucurbit[10]uril, see Liu, S.; Zavalij, P.Y.; Isaacs, L. J. Am. Chem. Soc. 2005, 127, 16798.