March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 4. Stereochemistry and Conformation
The discussions in Chapters 1–3 focused on electron distribution in organic molecules. In this chapter, the focus will be on the 3D structure of organic compounds.1 The structure may be such that stereoisomerism2 is possible. Stereoisomers are compounds made up of the same atoms bonded by the same sequence of bonds, but having different 3D structures that are not interchangeable. These structures are called configurations.
4.A. Optical Activity and Chirality3
Any material that rotates the plane of polarized light is said to be optically active. If a pure compound is optically active, the molecule is nonsuperimposable on its mirror image. If a molecule is superimposable on its mirror image, the two structures constitute the same compound and the compound does not rotate the plane of polarized light; it is optically inactive. The property of nonsuperimposability of an object on its mirror image is called chirality. If a molecule is not superimposable on its mirror image, it is chiral. If it is superimposable on its mirror image, it is achiral. The relationship between optical activity and chirality is absolute. No exceptions are known, and many thousands of cases have been found in accord with it (however, see Sec. 4.C). The ultimate criterion, then, for optical activity is chirality (nonsuperimposability on the mirror image). This finding is both a necessary and a sufficient condition.4 This fact has been used as evidence for the structure determination of many compounds, and historically the tetrahedral nature of carbon was deduced from the hypothesis that the relationship might be true. Note that parity violation represents an essential property of particle and atomic handedness, and has been related to chirality.5
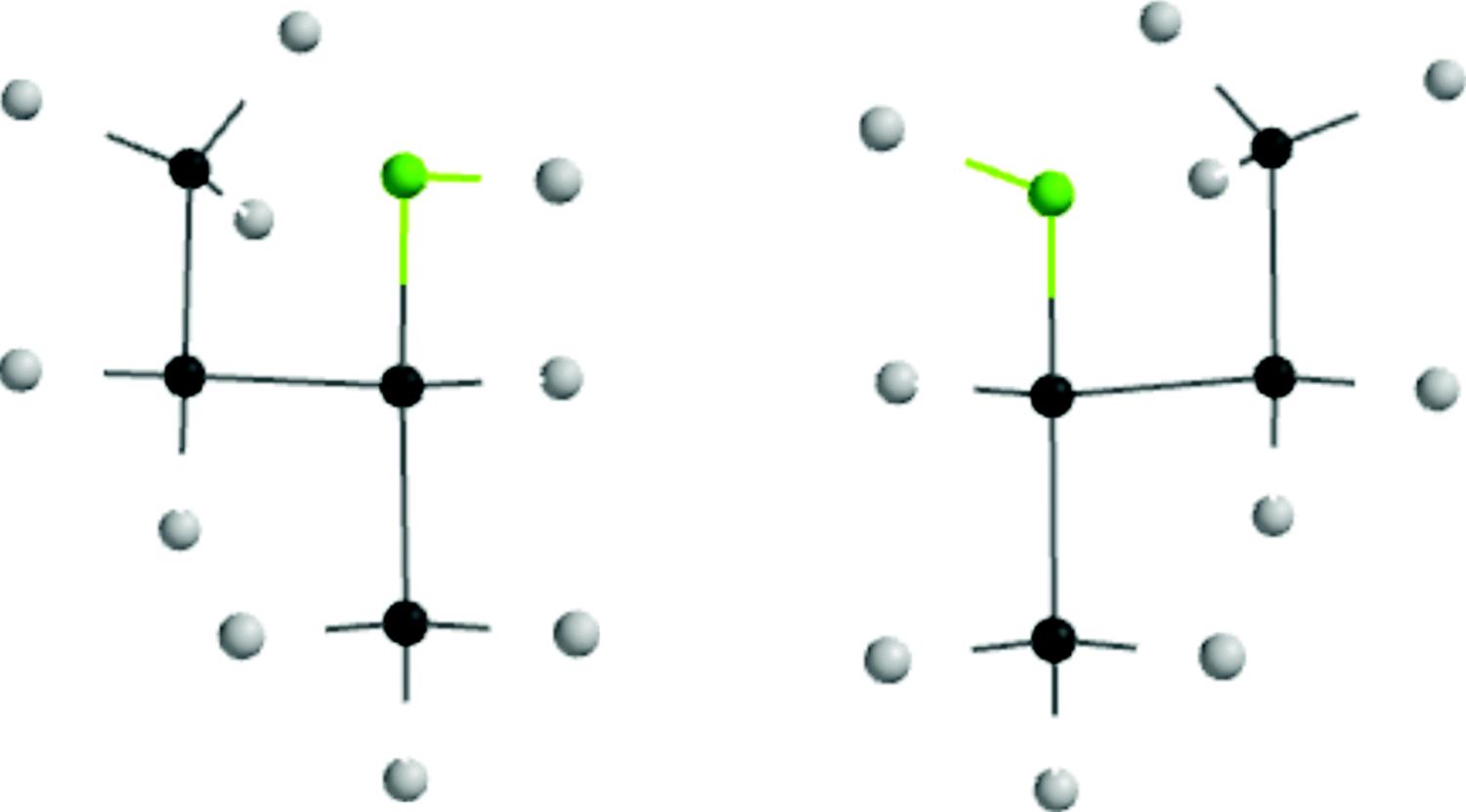
If a molecule is nonsuperimposable on its mirror image, the mirror image must be a different molecule, since superimposability is the same as identity. In each case of optical activity of a pure compound there are two and only two isomers, called enantiomers (sometimes enantiomorphs), which differ in structure only in the left- and right-handedness of their orientations (see the enantiomers for 2-butanol in Fig. 4.1). Enantiomers have identical6 physical and chemical properties except in two important respects:
1. They rotate the plane of polarized light in opposite directions, although in equal amounts. The isomer that rotates the plane to the left (counterclockwise) is called the levo isomer and is designated (−), while the one that rotates the plane to the right (clockwise) is called the dextro isomer and is designated (+). Because they differ in this property they are often called optical antipodes.
2. They may react at different rates with other chiral compounds. These rates may be so close together that the distinction is practically useless, or they may be so far apart that one enantiomer undergoes the reaction at a convenient rate while the other does not react at all. This finding is the reason that many compounds are biologically active while their enantiomers are not. Enantiomers react at the same rate with achiral compounds.7
Fig. 4.1 Enantiomers of 2-butanol.
In general, it may be said that enantiomers have identical properties in a symmetrical environment, but their properties may differ in an unsymmetrical environment.8 Besides the important differences previously noted, enantiomers may react at different rates with achiral molecules if an optically active catalyst is present; they may have different solubilities in an optically active solvent; they may have different indexes of refraction or absorption spectra when examined with circularly polarized light, and so on. In most cases, these differences are too small to be useful and are often too small to be measured.
Although pure compounds are always optically active if they are composed of chiral molecules, mixtures of equal amounts of enantiomers are optically inactive since the equal and opposite rotations cancel. Such mixtures are called racemic mixtures9 or racemates.10 Their properties are not always the same as those of the individual enantiomers. The properties in the gaseous or liquid state or in solution usually are the same, since such a mixture is nearly ideal, but properties involving the solid state11 (e.g., melting points, solubilities, and heats of fusion), are often different. Thus racemic tartaric acid has a melting point of 204–206 °C and a solubility in water at 20 °C of 206 g L−1while for the (+) or the (−) enantiomer, the corresponding figures are 170 °C and 1390 g L−1. The separation of a racemic mixture into its two optically active components is called resolution. The presence of optical activity always proves that a given compound is chiral, but its absence does not prove that the compound is achiral. A compound that is optically inactive may be achiral, or it may be a racemic mixture (see also, Sec. 4.C).
4.A.i. Dependence of Rotation on Conditions of Measurement
The amount of rotation α is not a constant for a given enantiomer; it depends on the length of the sample vessel, the temperature, the solvent12 and concentration (for solutions), the pressure (for gases), and the wavelength of light.13Of course, rotations determined for the same compound under the same conditions are identical. The length of the vessel and the concentration or pressure determine the number of molecules in the path of the beam, and α is linear with this. To make it possible for one value of α for a pure compound to be compared with another α for that compound taken under different circumstances, a physical property is defined, called the specific rotation [α], which is
![]()
where α is the observed rotation, l is the cell length in decimeters, c is the concentration in grams per milliliter, and d is the density in the same units. The specific rotation is usually given along with the temperature and wavelength of light used for the measurement, in this manner: ![]() . These conditions must be duplicated for comparison of rotations, since there is no way to put them into a simple formula. The expression [α]D means that the rotation was measured with sodium D light; that is, λ = 589 nm. The molar rotation
. These conditions must be duplicated for comparison of rotations, since there is no way to put them into a simple formula. The expression [α]D means that the rotation was measured with sodium D light; that is, λ = 589 nm. The molar rotation ![]() is the specific rotation times the molecular weight divided by 100.
is the specific rotation times the molecular weight divided by 100.
It must be emphasized that the value of α changes with conditions, but the molecular structure is unchanged. This finding is true even when the changes in conditions are sufficient to change not only the amount of rotation, but even the direction. Thus one of the enantiomers of aspartic acid, when dissolved in water, has [α]D equal to +4.36° at 20°C and −1.86° at 90 °C, although the molecular structure is unchanged. A consequence of such cases is that there is a temperature at which there is no rotation (in this case 75°C). Of course, the other enantiomer exhibits opposite behavior.
Other cases are known in which the direction of rotation is reversed by changes in wavelength, solvent, and even concentration.14 In theory, there should be no change in [α] with concentration, since this is taken into account in the formula, but associations, dissociations, and solute–solvent interactions often cause nonlinear behavior. For example, ![]() for (−)-2-ethyl-2-methylsuccinic acid in CHCl3 is −5.0° at c = 16.5 g 100 mL−1 (0.165 g mL−1), −0.7° at c = 10.6, +1.7° at c = 8.5, and +18.9° at c = 2.2.15 Note that the concentration is sometimes reported in g 100 mL−1 (as shown) or as g dL−1 (decaliters) rather than the standard g mL−1. One should always check the concentration term to be certain. Note that calculation of the optical rotation of (R)-(−)-3-chloro-1-butene found a remarkably large dependence on the C=C–C–C torsional angle.16 However, the observed rotations are a factor of 2.6 smaller than the calculated values, independent of both conformation and wavelength from 589 to 365 nm.
for (−)-2-ethyl-2-methylsuccinic acid in CHCl3 is −5.0° at c = 16.5 g 100 mL−1 (0.165 g mL−1), −0.7° at c = 10.6, +1.7° at c = 8.5, and +18.9° at c = 2.2.15 Note that the concentration is sometimes reported in g 100 mL−1 (as shown) or as g dL−1 (decaliters) rather than the standard g mL−1. One should always check the concentration term to be certain. Note that calculation of the optical rotation of (R)-(−)-3-chloro-1-butene found a remarkably large dependence on the C=C–C–C torsional angle.16 However, the observed rotations are a factor of 2.6 smaller than the calculated values, independent of both conformation and wavelength from 589 to 365 nm.