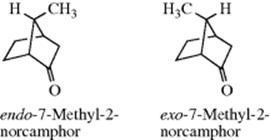March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 4. Stereochemistry and Conformation
4.J. cis–trans Isomerism
Compounds in which rotation is restricted may exhibit cis–trans isomerism.225 These compounds do not rotate the plane of polarized light (unless they also happen to be chiral), and the properties of the isomers are not identical. The two most important types are isomerism resulting from double bonds and that resulting from rings.
4.J.i. cis-trans Isomerism Resulting from Double Bonds
It has been mentioned (Sec. 1.D) that the two carbon atoms of a C=C d=ble bond and the four atoms directly attached to them are all in the same plane and that the presence of the π-bond prevents rotation around the double bond. This means that in the case of a molecule WXC=CYZ, stereoisomerism exists when W ≠ X and Y ≠ Z. There are two and only two isomers (62 and 63), each superimposable on its mirror image unless one of the groups happens to carry a stereogenic center. Note that 62 and 63 are diastereomers, by the definition given in Section 4.E.i. There are two ways to name such isomers. In the older and less versatile method, one isomer is called cis and the other trans. When each carbon of the C=C unit has an identical group (W in 62 and 63), but fits the substitution pattern of 62 and 63, the cis-trans nomenclature system may be applied. When the two identical groups are on the same side (W and W in 62), it is labeled cis. cis-3-Hexene is shown as an example. When the two identical groups are on opposite side (W and W in 63) it is labeled trans. trans-3-Hexene is shown as an example. Unfortunately, there is no obvious way to apply this method when the four groups are different.
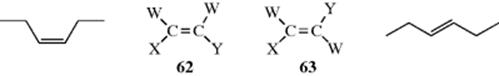
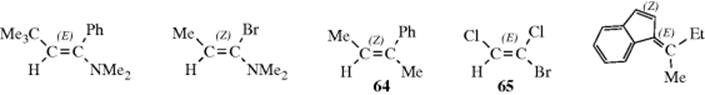
The newer and more widely applicable method can be applied to all cases, and is based on the CIP system (Sec. 4.E.i). The two groups at each carbon of the C=C unit are ranked by the sequence rules. The isomer with the two higher ranking groups on the same side of the double bond is called (Z) (for the German word zusammen meaning together). The isomer with the two higher ranking groups on opposite sides of the double bond is called (E) (for entgegen meaning opposite).226 A few examples are shown. Note that the (Z) isomer is not necessarily the one that would be called cis under the older system (e.g., 64 and 65). Like cis and trans, (E) and (Z) are used as prefixes; for example, 65 is called (E)-1-bromo-1,2-dichloroethene.
This type of isomerism is also possible with other double bonds (e.g., C=N,227 N=N,228 or even C=S),229 although in these cases only two or three groups are connected to the double-bond atoms. In the case of imines, oximes, and other C=N compounds, if W = Y, 66 may be called syn and 67 anti, but (E) and (Z) are used here too.230 In azo compounds there is no ambiguity. Compound 68 is always syn or (Z) regardless of the nature of W and Y.
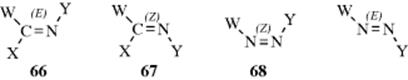
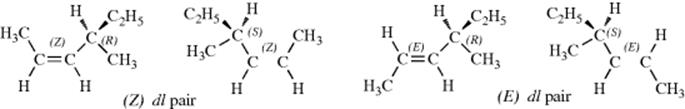
If there is more than one double bond231 in a molecule and if W ≠ X and Y ≠ Z for each, the number of isomers in the most general case is 2n, although this number may be decreased if some of the substituents are the same, as in the three 2,5-heptadienes shown.
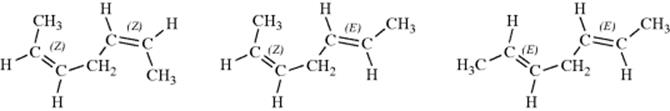
When a molecule contains a double bond and a stereogenic carbon, there are four isomers, a cis pair of enantiomers and a trans pair, shown for 4-methylhex-2-ene.
Double bonds in small rings are so constrained that they must be cis. From cyclopropene (a known system) to cycloheptene, double bonds in a stable ring cannot be trans. However, the cyclooctene ring is large enough to permit trans double bonds to exist (see Sec. 4.C, category 7), and for rings larger than 10- or 11-membered, trans isomers are more stable232 (see also, Sec. 4.Q.ii).
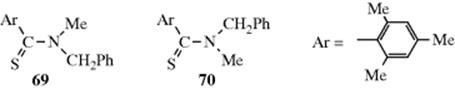
In a few cases, single-bond rotation is so slowed that cis and trans isomers can be isolated even where no double bond exists233 (see also, Sec. 4.Q.iv). One example is N-methyl-N-benzylthiomesitylide (69 and 70).234 The isomers are stable in the crystalline state, but interconvert with a half-life of ~25 h in CDCl3 at 50°C.235 This type of isomerism is rare; it is found chiefly in certain amides and thioamides, because resonance gives the single-bond some double-bond character and slows rotation.54 (For other examples of restricted rotation about single bonds, see Sec. 4.Q.iv.)

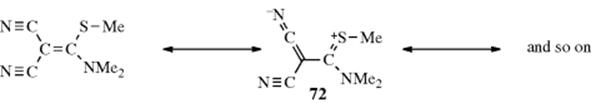
Conversely, there are compounds in which nearly free rotation is possible around what are formally C=C double bonds. These compounds, called push–pull or captodative ethylenes, have two electron-withdrawing groups on one carbon and two electron-donating groups on the other (71).236 The contribution of diionic
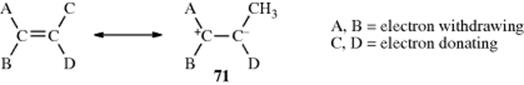
canonical forms, such as the one shown, decreases the double-bond character and allows easier rotation. For example, compound 72 has a barrier to rotation of 13 kcal mol−1 (55 kJ mol−1),237 compared to a typical value of ~62–65 kcal mol−1(260–270 kJ mol−1) for simple alkenes.
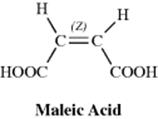
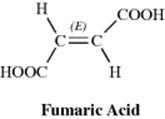
Since they are diastereomers, cis-trans isomers always differ in properties; the differences may range from very slight to considerable. The properties of maleic acid are so different from those of fumaric acid (Table 4.2) that it is not surprising that they have different names. Since they generally have more symmetry than cis isomers, trans isomers in most cases have higher melting points and lower solubilities in inert solvents. The cis isomer usually has a higher heat of combustion, which indicates a lower thermochemical stability. Other noticeably different properties are densities, acid strengths, boiling points, and various types of spectra, but the differences are too involved to be discussed here.
Table 4.2 Some Properties of Maleic and Fumaric Acids.
Property
Maleic Acid
Fumaric Acid
Melting point (°C)
130
286
Solubility in water at 25 °C (g L−1)
788
7
K1 (at 25°C)
1.5 × 10−2
1 × 10−3
K2 (at 25°C)
2.6 × 10−7
3 × 10−5
It is also important to note that trans-alkenes are often more stable than cis-alkenes due to diminished steric hindrance (Sec. 4.Q.iv), but this is not always the case. It is known, for example, that cis-1,2-difluoroethene is thermodynamically more stable than trans-1,2-difluoroethene. This appears to be due to delocalization of halogen lone-pair electrons and an antiperiplanar effect between vicinal antiperiplanar bonds.238
4.J.ii cis–trans Isomerism of Monocyclic Compounds
Although rings of four carbons and larger are not generally planar (see Sec. 4.O), they will be treated as such in this section, since the correct number of isomers can be determined when this is done239 and the principles are easier to visualize (see Sec. 4.O).
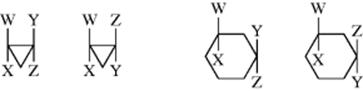
The presence of a ring, like that of a double bond, prevents rotation. cis and trans isomers are possible whenever there are two carbons on a ring, each of which is substituted by two different groups. The two carbons need not be adjacent. Examples follow:
In some cases, the two stereoisomers can interconvert. In cis- and trans-disubstituted cyclopropanones, for example, there is reversible interconversion that favors the more stable trans isomer. This fluxional isomerization occurs via ring opening to an unseen oxyallyl valence bond isomer.240
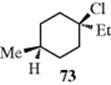
While cis and trans isomers are possible for rings, the restrictions are that W may equal Y and X may equal Z, but W may not equal X and Y may not equal Z. There is an important difference from the double-bond case: The substituted carbons may be stereogenic carbons. This means that there may be more than two isomers. In the most general case, where W, X, Y, and Z are all different, there are four isomers since neither the cis nor the trans isomer is superimposable on its mirror image. This is true regardless of ring size or which carbons are involved, except that in rings of even-numbered size when W, X, Y, and Z are at opposite corners. Cyclohexane derivative 73, for example, has no stereogenic carbons because there is a plane of symmetry. Imagine a focus on the chlorine-bearing carbon, and view each “arm” of the ring as a group. There are two identical groups so the carbon will not be stereogenic. When W = Y and X = Z, the cis isomer is always superimposable on its mirror image. Hence, this isomer is a meso compound, while the trans isomer consists of a dl pair, except in the case noted above. Again, the cis isomer has a plane of symmetry while the trans does not.
Rings with more than two differently substituted carbons can be dealt with using similar principles. In some cases, it is not easy to tell the number of isomers by inspection.107 The best method may be to count the number n of differently substituted carbons (these will usually be asymmetric, but not always, e.g., in 73), and then to draw 2n structures, crossing out those that can be superimposed on others (usually the easiest method is to look for a plane of symmetry). By this means, it can be determined that for 1,2,3-cyclohexanetriol there are two meso compounds and a dl pair; and for 1,2,3,4,5,6-hexachlorocyclohexane there are seven meso compounds and a dl pair. Similar principles apply to heterocyclic rings as long as there are carbons (or other ring atoms) containing two different groups.
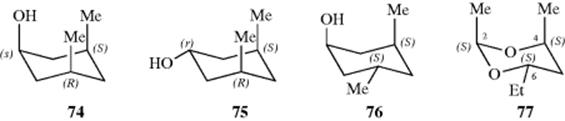
Cyclic stereoisomers containing only two differently substituted carbons are named either cis or trans, as previously indicated. The (Z, E) system is not used for cyclic compounds. However, cis-trans nomenclature will not suffice for compounds with more than two differently substituted atoms. For these compounds, a system is used in which the configuration of each group is given with respect to a reference group, which is chosen as the group attached to the lowest-numbered ring member bearing a substituent giving rise to cis-trans isomerism. The reference group is indicated by the symbol r. Three stereoisomers named according to this system are 3(S), 5(R)-dimethylcyclohexan-s-1-ol (74), 3(S), 5(R)-dimethylcyclohexan-r-1-ol (75), and 3(S), 5(S)-dimethylcyclohexan-s-1-ol (76). The last example demonstrates the rule that when there are two otherwise equivalent ways of going around the ring, one chooses the path that gives the cis designation to the first substituent after the reference. Another example is 2(S), 4(S)-dimethyl-6s-ethyl-1,3-dioxane (77).
4.J.iii. cis–trans Isomerism of Fused and Bridged Ring Systems
Fused bicyclic systems are those in which two rings share two and only two atoms. In such systems, there is no new principle. The fusion may be cis or trans, as illustrated by cis- and trans-decalin. However, when the rings are small enough, the trans configuration is impossible and the junction must be cis. The smallest trans junction that has been prepared when one ring is four membered is a four–five junction; trans-bicyclo[3.2.0]heptane (78) is known.241 For the bicyclo[2.2.0] system (a four–four fusion), only cis compounds have been made. The smallest known trans junction when one ring is three membered is a six–three junction (a bicyclo[4.1.0] system). An example is 79.242 When one ring is three-membered and the other eight-membered (an eight–three junction), the trans-fused isomer is more stable than the corresponding cis-fused isomer.243
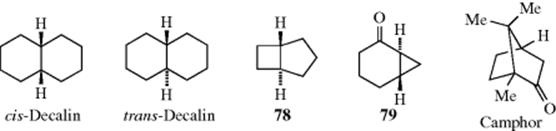
In bridged bicyclic ring systems, two rings share more than two atoms. In these cases, there may be fewer than 2n isomers, because of the structure of the system. For example, there are only two isomers of camphor (a pair of enantiomers), although it has two stereogenic carbons. In both isomers, the methyl and hydrogen are cis. The trans pair of enantiomers is impossible in this case, since the bridge must be cis. The smallest bridged system so far prepared in which the bridge is trans is the [4.3.1] system; the trans ketone (80) has been prepared.244 In this case, there are four isomers, since both the trans and the cis, which has also been prepared, are pairs of enantiomers.
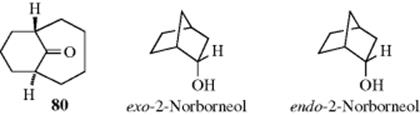
When one of the bridges contains a substituent, the question arises as to how to name the isomers involved. When the two bridges that do not contain the substituent are of unequal length, the rule generally followed is that the prefix endo- is used when the substituent is closer to the longer of the two unsubstituted bridges; the prefix exo- is used when the substituent is closer to the shorter bridge; for example, When the two bridges not containing the substituent are of equal length, this convention cannot be applied, but in some cases a decision can still be made. For example, if one of the two bridges contains a functional group, the endo isomer is the one in which the substituent is closer to the functional group: