March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 4. Stereochemistry and Conformation
4.K. Out–In Isomerism
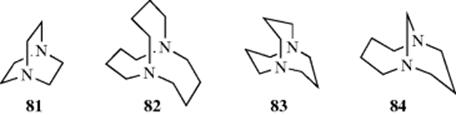
Another type of stereoisomerism, called out–in isomerism (or in–out),245 is found in salts of tricyclic diamines with nitrogen at the bridgeheads. In medium-sized bicyclic ring systems, in–out isomerism is possible,246 and the bridgehead nitrogen atoms adopt the arrangement that is more stable.247 A focus on the nitrogen lone pairs reveals that 1,4-diazabicyclo[2.2.2]octane (81) favors the out–out isomer, that 1,6-diazabicyclo[4.4.4]tetradecane (82) the in–in,248 that 1,5-diazabicyclo[3.3.3]undecane (83) has nearly planar nitrogen atoms,249 and that 1,9-diazabicyclo[7.3.1]tridecane (84) is in–out.250 One can also focus on the NH unit in the case of ammonium salts.
In the examples 85–87, when k, l, and m > 6, the N–H bonds can be inside the molecular cavity or outside, giving rise to three isomers, as shown. Simmons and Park251 isolated several such isomers with k, l, and m varying from 6 to 10. In the 9,9,9 compound, the cavity of the in–in isomer is large enough to encapsulate a
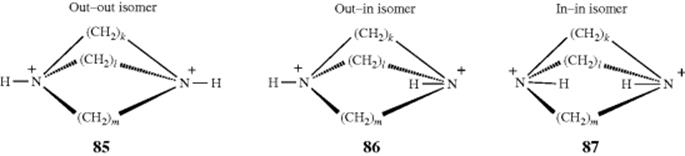
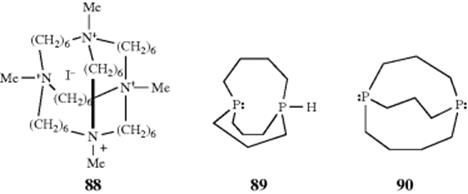
chloride ion that is hydrogen bonded to the two N–H groups. The species thus formed is a cryptate, but differs from the cryptates discussed at Section 3.C.ii in that there is a negative rather than a positive ion enclosed.252 Even smaller ones (e.g., the 4,4,4 compound) have been shown to form mono-inside-protonated ions.253 In compound 88, which has four quaternary nitrogen atoms, a halide ion has been encapsulated without a hydrogen being present on a nitrogen.254 This ion does not display in–out isomerism. Out–in and in–in isomers have also been prepared in analogous all-carbon tricyclic systems.255
It is known that chiral phosphanes are more pyramidal and that inversion is more difficult, usually requiring temperatures well over 100°C for racemization.256 Alder and Read257 found that deprotonation of bis(phosphorane) 89,which is known to have an in–out structure with significant P–P bonding, leads to a rearrangement and the out–out diphosphane 90. Reprotonation gives 89,258 with inversion at the nonprotonated phosphorus atom occurring at room temperature.