March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 5. Carbocations, Carbanions, Free Radicals, Carbenes, and Nitrenes
5.D. Carbenes
5.D.i. Stability and Structure348
Carbenes are highly reactive species, and practically all have lifetimes considerably < 1 s. With exceptions noted below (Sec. 5.D.ii), carbenes have been isolated only by entrapment in matrices at low temperatures (77 K or less).349The parent species (CH2) is usually called methylene, although derivatives are more often named by the carbene nomenclature. Thus CCl2 is generally known as dichlorocarbene, although it can also be called dichloromethylene.
The two nonbonded electrons of a carbene can be either paired or unpaired. If they are paired, the species is spectrally a singlet, while, as seen above (Sec. 5.C.i), two unpaired electrons appear as a triplet. An ingenious

method of distinguishing between the two possibilities was developed by Skell,350 based on the common reaction of addition of carbenes to double bonds to form cyclopropane derivatives (Reaction 15-51). If the singlet species adds to cis-2-butene, the resulting cyclopropane should be the cis isomer since the movements of the two pairs of electrons
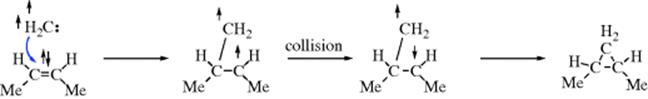
should occur either simultaneously or with one rapidly succeeding another. However, if the attack is by a triplet species, the two unpaired electrons cannot both go into a new covalent bond, since by Hund's rule they have parallel spins. So one of the unpaired electrons will form a bond with the electron from the double bond that has the opposite spin, leaving two unpaired electrons that have the same spin and therefore cannot form a bond at once, but must wait until, by some collision process, one of the electrons can reverse its spin. During this time, there is free rotation about the C–C bond and a mixture of cis- and trans-1,2-dimethylcyclopropanes should result.351
The results of this type of experiment show that CH2 itself is usually formed as a singlet species, which can decay to the triplet state, which consequently has a lower energy (MO calculations352 and experimental determinations show that the difference in energy between singlet and triplet CH2 is ~8–10 kcal mol−1 or 33–42 kJ mol−1)353. However, it is possible to prepare triplet CH2 directly by a photosensitized decomposition of diazomethane.354 The CH2group is so reactive355 that it generally reacts as the singlet before it has a chance to decay to the triplet state.356 As to other carbenes, some react as triplets, some as singlets, and others as singlets or triplets, depending on how they are generated. There are, however, molecules that generate persistent triplet carbenes.357 Indeed, remarkably stable diaryl triplet carbenes have been prepared,358 and protected diphenylcarbenes are particularly stable.359 There are also persistent singlet carbenes, although radical fragmentation is a problem.360
There is a limitation to the use of stereospecificity of addition as a diagnostic test for singlet or triplet carbenes.361 When carbenes are generated by photolytic methods, they are often in a highly excited singlet state. When they add to the double bond, the addition is stereospecific; but the cyclopropane formed carries excess energy (i.e., it is in an excited state). It has been shown that under certain conditions (low pressures in the gas phase) the excited cyclopropane may undergo cis–trans isomerization after it is formed, so that triplet carbene may seem to be involved although in reality the singlet was present.362
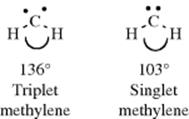
Studies of the IR spectrum of CCl2 trapped at low temperatures in solid argon indicate that the ground state for this species is the singlet.363 The geometrical structure of triplet methylene can be investigated by ESR measurements,364 since triplet species are diradicals. Such measurements made on triplet CH2 trapped in matrices at very low temperatures (4 K) show that triplet CH2 is a bent molecule, with an angle of ~136°.365 The EPR measurements cannot be made on singlet species, but from electronic spectra of CH2 formed in flash photolysis366 of diazomethane it was concluded that singlet CH2 is also bent, with an angle of ~103°.367 Singlet CCl2300 and CBr2368 are also bent, with angles of 100 and 114°, respectively. It has long been known that triplet aryl carbenes are bent.369
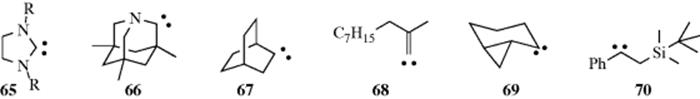
The most common carbenes are :CH2 and :CCl2,370 but many others have been reported,371 including heterocyclic carbenes372 diboron carbenes,373 65 (stabilized by the steric constraints of the ring geometry),374 66 (an aminocarbene without π conjugation),375 bicyclo[2.2.2]octylidene, (67),376 alkylidene carbenes (e.g., 68),377 conformationally restricted cyclopropylcarbenes, (e.g., 69),378 β-silylcarbenes (e.g., 70),379 α-keto carbenes,380 vinyl carbenes,381 and chiral carbenoids.382 Fluoro(phenoxy)carbene is stable for several days if it is generated within the cavity of a hemicarcerand (see Sec. 3.C.iii).383 In the case of 65 (R = Ph),384 the precursor is a tetraaminoethylene, and when potassium hydride is present to preclude electrophilic catalysis, starting tetraaminoethylenes are recovered unchanged.
Flash photolysis of CHBr3 produced the intermediate CBr,385 which is a carbyne.
![]()
The intermediates CF and CCl were generated similarly from CHFBr2 and CHClBr2, respectively. Triplet acetylenes have been reported as equivalents for 1,2-bicarbenes.386
5.D.ii. The Generation and Fate of Carbenes387
There are two primary methods to form carbenes, although other pathways are also known.
1. In α elimination, a carbon loses a group without its electron pair, usually a proton, and then a group with its pair, usually a halide ion:388
![]()
The most common example is formation of dichlorocarbene by treatment of chloroform with a base (see Reaction 10-3) and geminal alkyl dihalides with Me3Sn−,389 but many other examples are known, such as
![]()
Ref. 390
Ref. 391
2. Disintegration of compounds containing certain types of double bonds:
![]()
The two most important ways of forming:CH2 are examples: the photolysis of ketene
![]()
and the isoelectronic decomposition of diazomethane.392
![]()
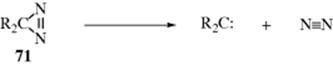
Some diazoalkanes decompose to the corresponding carbene.393 Diazirines394 (isomeric with diazoalkanes) give carbenes,395 but arylmethyl radicals have also been generated from diazirines.396 In a different study, thermolysis of diaryloxydiazirines (71) gave the anticipated carbene products, but photolysis gave both carbenes and aryloxy radicals by α-scission.397
Because most carbenes are so reactive, it is often difficult to prove that they are actually present in a given reaction. The lifetime of formylcarbene was measured to be 0.15–0.73 ns by transient absorption and transient grating spectroscopy in dichloromethane.398 In many instances, where a carbene is apparently produced by an α elimination or by disintegration of a double-bond compound, there is evidence that no free carbene is actually involved. The neutral term carbenoid is used where it is known that a free carbene is not present or in cases where there is doubt. α-Halo organometallic compounds, (R2CXM) are often called carbenoids because they readily give elimination reactions399 (e.g., see Reaction 12-39).
The reactions of carbenes are more varied than those of the species previously discussed in this chapter.400 Solvent effects have been observed in carbene reactions. The selectivity of certain carbenes is influenced by the nature of the solvent.401 The distribution of rearrangement products (see below) from tert-butylcarbene402 are influenced by changes in solvent.403 It is known that singlet methylene forms a charge-transfer complex with benzene.404 Solvent interactions for chlorophenylcarbene and fluorophenylcarbene, however, are weak.405
1. Additions to carbon–carbon double bonds have already been mentioned. Carbenes also add to aromatic systems, but the immediate products rearrange, usually with ring enlargement (see Reaction 15-65). Additions of carbenes to other double bonds [e.g., C=N (Reactions 16-46 and 16-48), and to triple bonds], have also been reported.
2. An unusual reaction of carbenes is that of insertion into C–H bonds (Reaction 12-21). Thus :CH2 reacts with methane to give ethane and with propane
![]()
to give n-butane and isobutane, as shown. Elimination to give an alkene is a competing side reaction in polar solvents, but this is suppressed in nonpolar solvents.406 Simple alkyl carbenes, such as this, are not very useful for synthetic purposes, but do illustrate the extreme reactivity of carbene. However, carbenoids generated by rhodium-catalyzed decomposition of diazoalkanes are very useful (see Reaction 12-23) and have been used in a variety of syntheses. Treatment in the liquid phase of an alkane (e.g., pentane) with carbene formed from the photolysis of diazomethane, gives the three possible products in statistical ratios407 demonstrating that carbene is displaying no selectivity. For many years, it was a generally accepted principle that the lower the selectivity the greater the reactivity; however, this principle is no longer regarded as general because many exceptions have been found.408Singlet CH2 generated by photolysis of diazomethane is probably the most reactive organic species known, but triplet CH2 is somewhat less reactive, and other carbenes are still less reactive. The following series of carbenes of decreasing reactivity has been proposed on the basis of discrimination between insertion and addition reactions: CH2 > HCCOOR > PhCH > BrCH ~ ClCH.409 Dihalocarbenes generally do not give insertion reactions at all. Insertion of carbenes into other bonds has also been demonstrated, although not insertion into C–C bonds.410
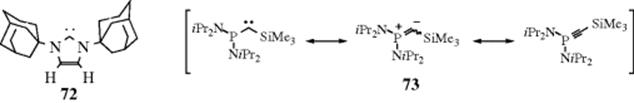
Two carbenes that are stable at room temperature have been reported:411 72 and 73. In the absence of oxygen and moisture, 72 exists as stable crystals with a melting point of 240–241 °C.412 This structure was proved by X-ray crystallography.
3. It would seem that dimerization to form an alkene should be an important reaction of carbenes, but it is not.
![]()
Generally, the reactivity is so great that the carbene species do not have time to find each other and because the dimer generally has so much energy that it dissociates again. Apparent dimerization has been observed, but it is likely that the products in many reported instances of “dimerization” do not arise from an actual dimerization of two carbenes, but come from attack by a carbene on a molecule of a carbene precursor, for example,
![]()
4. Alkylcarbenes can undergo rearrangement, with migration of alkyl or hydrogen.413 Indeed these rearrangements are generally so rapid414 that additions to multiple bonds and insertion reactions, which are so common for CH2, are seldom encountered with alkyl or dialkyl carbenes. Unlike rearrangement of the species previously encountered in this chapter, most rearrangements of carbenes directly give stable molecules. A carbene intermediate has been suggested for the isomerization of cyclopropane.415 Some examples of carbene rearrangement are

Ref. 416
![]()
Ref. 417

Ref. 418
![]()
Ref. 419
The rearrangement of acylcarbenes to ketenes is called the Wolff rearrangement (Reaction 18-8). A few rearrangements in which carbenes rearrange to other carbenes are also known.420 Of course, the new carbene must stabilize itself in one of the ways mentioned.
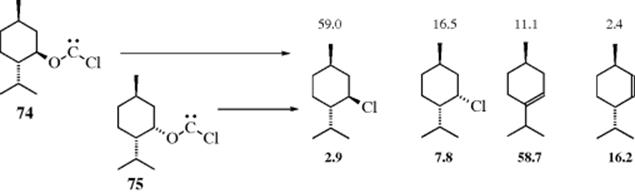
5. The fragmentation reactions of alicyclic oxychlorocarbenes (e.g., 74 and 75)421 give substitution and elimination products. Menthyloxychlorocarbene (74) gave primarily the substitution product, whereas neomenthyloxychlorocarbene (75) gave primarily the elimination product, as shown. In this case, the substitution product is likely due to rearrangement of the chlorocarbene.422 It is known that fragmentation of nortricyclyloxychlorocarbene in pentane occurs by an SNi-like process to give nortricyclyl chloride.423 In more polar solvents, fragmentation leads to nortricyclyl cation–chloride anion pair that gives nortricyclyl chloride and a small amount of exo-2-norbornenyl chloride. Fragmentation can also lead to radicals.424
6. Triplet carbenes can abstract hydrogen or other atoms to give free radicals, for example,
![]()
This is not surprising, since triplet carbenes are free radicals. But singlet carbenes425 can also give this reaction, although in this case only halogen atoms are abstracted, not hydrogen.426