March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 8. Acids and Bases
8.B. The Mechanism of Proton-Transfer Reactions
Proton transfers between a base and an oxygen or nitrogen acid are usually extremely fast.108 Such reactions are generally diffusion controlled in the thermodynamically favored direction.109 In fact, a normal acid is defined110 as one whose proton-transfer reactions are completely diffusion controlled, except when the conjugate acid of the base to which the proton is transferred has a pK value very close (differs by less than ~ 2 pK units) to that of the acid. The normal acid–base reaction mechanism consists of three steps:
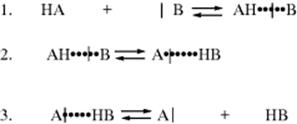
The actual proton transfer takes place in the second step the first step is formation of a hydrogen-bonded complex. The product of the second step is another hydrogen-bonded complex, which dissociates in the third step.
However, not all such proton transfers are diffusion controlled. For example, if an internal hydrogen bond exists in a molecule, reaction with an external acid or base is often much slower.111 In a case such as 3-hydroxypropanoic acid, the −OH ion can form a hydrogen bond with the acidic hydrogen only if the internal hydrogen bond breaks. Therefore only some of the collisions between −OH ions and 3-hydroxypropanoic acid molecules result in proton transfer. In many collisions, the −OH ions will come away “empty-handed”, resulting in a lower reaction rate. Note that this affects only the rate, not the equilibrium. Other systems are capable of hydrogen bonding (e.g., 1,2-diols). In the case of cyclohexane-1,2-diols, hydrogen bonding, ion–dipole interactions, polarizability, and stereochemistry all play a role in determining the acidity.112 The presence of halogen atoms (e.g., chlorine) can lead to hydrogen-bonding effects.113 Another factor that can create lower rates is a molecular structure in which the acidic proton is protected within a molecular cavity (e.g., the in–in and out–in isomers shown in Sec. 4.L). See also the proton sponges mentioned in Section 8.F. Proton transfers between an acidic and a basic group within the same molecule can also be slow, if the two groups are too far apart for hydrogen bonding. In such cases, participation of solvent molecules may be necessary.
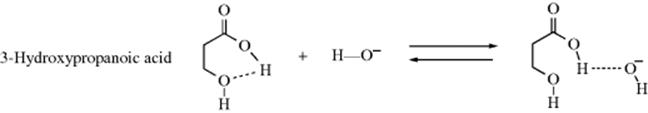
Proton transfers to or from a carbon atom114 in most cases are much slower than those strictly between oxygen or nitrogen atoms. At least three factors can be responsible for this,115 not all of them applying in every case.
1. Hydrogen bonding is very weak or altogether absent for carbon (Chap 3).
2. Loss of a proton from many carbon acids leads to carbanions that are stabilized by resonance. Calculations show that carbon acidity is influenced by coordination based on electrophile coordination geometry.116 Structural reorganization (movement of atoms to different positions within the molecule) may accompany this process. Chloroform, HCN, and 1-alkynes do not form resonance-stabilized carbanions, and these117 behave kinetically as normal acids.118 It has been reported that carborane acids [e.g., H(CHB11H5Cl6)] are the strongest isolable (Lewis-free) Br![]() nsted acids known.119
nsted acids known.119
3. There may be considerable reorganization of solvent molecules around the ion as compared to the neutral molecule.120
In connection with factors 2 and 3, it has been proposed115 that any factor that stabilizes the product (e.g., by resonance or solvation) lowers the rate constant if it develops late on the reaction coordinate, but increases the rate constant if it develops early. This is called the Principle of Imperfect Synchronization.
Mechanisms of proton transfer have been studied for many compounds, including the reactions of acids with lactams,121 amides with various bases,122 and amines with alkoxide bases.123