March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 8. Acids and Bases
8.D. Acid and Base Catalysis148
Many reactions are catalyzed by acids or bases. Some are catalyzed by both acids and bases. In such cases, the catalyst is involved in a fundamental way in the mechanism. The first step of such a reaction is nearly always a proton transfer between the catalyst and the substrate.
Reactions can be catalyzed by acid or base in two different ways, called general and specific catalysis. If the rate of an acid-catalyzed reaction run in a solvent (S) is proportional to its conjugate acid [SH+], the reaction is said to be subject to specific acid catalysis, the acid being the lyonium ion (SH+). The acid that is put into the solvent may be stronger or weaker than SH+, but the rate is proportional only to the [SH+] that is actually present in the solution derived from the equilbrium
![]()
The identity of HA is important only to the extent that it determines the position of equilibrium, and hence the [SH+]. Most measurements have been made in water, where SH+ is H3O+.
In general acid catalysis, the rate is increased not only by an increase in [SH+], but also by an increase in the concentration of other acids (e.g., in water by phenols or carboxylic acids). These other acids increase the rate even when [SH+] is held constant. In this type of catalysis the strongest acids catalyze best, so that, in the example given, an increase in the phenol concentration catalyzes the reaction much less than a similar increase in [H3O+]. This relationship between acid strength of the catalyst and its catalytic ability can be expressed by the Br![]() nsted catalysis equation149
nsted catalysis equation149
![]()
where k is the rate constant for a reaction catalyzed by an acid of ionization constant Kα. According to this equation, when log k is plotted against log Kα for catalysis of a given reaction by a series of acids, a straight line should be obtained with slope and intercept C. Straight lines are obtained in many cases, but not always. The relationship usually fails when acids of different types are compared. For example, it is much more likely to hold for a group of substituted phenols than for a collection of acids that contains both phenols150 and carboxylic acids. The Br![]() nsted equation is another linear free energy relationship (see Sec. 9.C).
nsted equation is another linear free energy relationship (see Sec. 9.C).
Analogously, there are general and specific (S− from an acidic solvent SH) base-catalyzed reactions. The Br![]() nsted law for bases is
nsted law for bases is
![]()
The Br![]() nsted equations relate a rate constant k to an equilibrium constant Ka. In Chapter 6, the Marcus equation was seen to relate a rate term (in that case ΔG‡) to an equilibrium term (ΔG°). When the Marcus treatment is applied to proton transfers151 between a carbon and an oxygen (or a nitrogen), the simplified152 equation (Sec. 6.I)
nsted equations relate a rate constant k to an equilibrium constant Ka. In Chapter 6, the Marcus equation was seen to relate a rate term (in that case ΔG‡) to an equilibrium term (ΔG°). When the Marcus treatment is applied to proton transfers151 between a carbon and an oxygen (or a nitrogen), the simplified152 equation (Sec. 6.I)
![]()
where
![]()
can be further simplified: Because proton transfers between oxygen and oxygen (or nitrogen and nitrogen) are much faster than those between carbon and carbon, ![]() is much smaller than
is much smaller than ![]() and one can write153
and one can write153
![]()
Thus, if the carbon part of the reaction is kept constant and only the A of HA is changed (where A is an oxygen or nitrogen moiety), then ΔG‡ is dependent only on ΔG°. Differentiation of this equation yields the Br![]() nsted α:
nsted α:
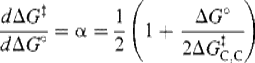
The Br![]() nsted law is therefore a special case of the Marcus equation.
nsted law is therefore a special case of the Marcus equation.
A knowledge of whether a reaction is subject to general or specific acid catalysis supplies information about the mechanism. For any acid-catalyzed reaction we can write
![]()
If the reaction is catalyzed only by the specific acid SH+, it means that step 1 is rapid and step 2 is rate controlling. This means that an equilibrium has been rapidly established between A and the strongest acid present in the solution, namely, SH+ (since this is the strongest acid that can be present in S). On the other hand, if step 2 is faster, there is no time to establish equilibrium and the rate-determining step must be step 1. This step is affected by all the acids that may be present, and the rate reflects the sum of the effects of each acid (general acid catalysis). General acid catalysis is also observed if the slow step is the reaction of a hydrogen-bond complex (A![]() HB), since each complex reacts with a base at a different rate. A comparable discussion can be used for general and specific base catalysis.154 Further information can be obtained from the values α and β in the Br
HB), since each complex reacts with a base at a different rate. A comparable discussion can be used for general and specific base catalysis.154 Further information can be obtained from the values α and β in the Br![]() nsted catalysis equations, since these are approximate measures of the extent of proton transfer in the transition state. In most cases, values of α and β are between 1 and 0. A value of α or β near 0 is generally taken to mean that the transition state resembles the reactants; that is, the proton has been transferred very little when the transition state has been reached. A value of α or β near 1 is taken to mean the opposite; that is, in the transition state the proton has been almost completely transferred. However, cases are known in which these generalizations are not followed,155 and their theoretical basis has been challenged.156 In general, the proton in the transition state lies closer to the weaker base.
nsted catalysis equations, since these are approximate measures of the extent of proton transfer in the transition state. In most cases, values of α and β are between 1 and 0. A value of α or β near 0 is generally taken to mean that the transition state resembles the reactants; that is, the proton has been transferred very little when the transition state has been reached. A value of α or β near 1 is taken to mean the opposite; that is, in the transition state the proton has been almost completely transferred. However, cases are known in which these generalizations are not followed,155 and their theoretical basis has been challenged.156 In general, the proton in the transition state lies closer to the weaker base.