March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 8. Acids and Bases
8.E. Lewis Acids and Bases
At about the same time that Br![]() nsted proposed his acid–base theory, Lewis put forth a broader theory. A base in the Lewis theory is the same as in the Br
nsted proposed his acid–base theory, Lewis put forth a broader theory. A base in the Lewis theory is the same as in the Br![]() nsted one, namely, a compound with an available pair of electrons, either unshared or in a π orbital. However, a Lewis base donates electrons to an atom other than H or C.157 A Lewis acid is any species with a vacant orbital.158 In a Lewis acid–base reaction, the unshared pair of the base forms a covalent bond with the vacant orbital of the acid, as represented by the general equation
nsted one, namely, a compound with an available pair of electrons, either unshared or in a π orbital. However, a Lewis base donates electrons to an atom other than H or C.157 A Lewis acid is any species with a vacant orbital.158 In a Lewis acid–base reaction, the unshared pair of the base forms a covalent bond with the vacant orbital of the acid, as represented by the general equation
![]()
in which charges are not shown, since they may differ. A specific example is
![]()
In the Br![]() nsted picture, the acid is a proton donor, but in the Lewis picture the proton itself is the acid since it has a vacant orbital. A Br
nsted picture, the acid is a proton donor, but in the Lewis picture the proton itself is the acid since it has a vacant orbital. A Br![]() nsted acid becomes, in the Lewis picture, the compound that gives up the actual acid. The advantage of the Lewis theory is that it correlates the behavior of many more processes. For example, AlCl3 and BF3 are Lewis acids because they have only six electrons in the outer shell and have room for eight. Lewis acids SnCl4and SO3 have eight, but their central elements, not being in the first row of the periodic table, have room for 10 or 12. Other Lewis acids are simple cations, like Ag+. The simple reaction
nsted acid becomes, in the Lewis picture, the compound that gives up the actual acid. The advantage of the Lewis theory is that it correlates the behavior of many more processes. For example, AlCl3 and BF3 are Lewis acids because they have only six electrons in the outer shell and have room for eight. Lewis acids SnCl4and SO3 have eight, but their central elements, not being in the first row of the periodic table, have room for 10 or 12. Other Lewis acids are simple cations, like Ag+. The simple reaction ![]() is not very common in organic chemistry, but the scope of the Lewis picture is much larger because reactions of the types shown here, which are very common in organic chemistry, are also Lewis acid–base reactions. In fact, all reactions in which a covalent bond is formed through one species contributing a filled and the other a vacant orbital may be regarded as Lewis acid–base reactions. An ab initio analysis of the factors that determine Lewis versus Lowry–Br
is not very common in organic chemistry, but the scope of the Lewis picture is much larger because reactions of the types shown here, which are very common in organic chemistry, are also Lewis acid–base reactions. In fact, all reactions in which a covalent bond is formed through one species contributing a filled and the other a vacant orbital may be regarded as Lewis acid–base reactions. An ab initio analysis of the factors that determine Lewis versus Lowry–Br![]() nsted acidity–basicity is available.159
nsted acidity–basicity is available.159

When a Lewis acid combines with a base to give a negative ion in which the central atom has a higher than normal valence, the resulting salt is called an ate complex.160 Examples are
![]()
Ate complexes are analogous to the onium salts formed when a Lewis base expands its valence, for example,
![]()
Far fewer quantitative measurements have been made of Lewis acid strength compared to that of Br![]() nsted acids.161 A simple table of Lewis acidities based on some quantitative measurement (e.g., that given for Br
nsted acids.161 A simple table of Lewis acidities based on some quantitative measurement (e.g., that given for Br![]() nsted acids in Table 8.1) is not feasible because Lewis acidity depends on the nature of the base and any solvent that can function as a base. For example, lithium perchlorate functions as a weak Lewis acid in ether.162 Qualitatively, the following approximate sequence of acidity of Lewis acids of the type MXn has been suggested, where X is a halogen atom or an inorganic radical: BX3 > AlX3 > FeX3 > GaX3 > SbX5 > SnX4 > AsX5 > ZnX2 > HgX2.
nsted acids in Table 8.1) is not feasible because Lewis acidity depends on the nature of the base and any solvent that can function as a base. For example, lithium perchlorate functions as a weak Lewis acid in ether.162 Qualitatively, the following approximate sequence of acidity of Lewis acids of the type MXn has been suggested, where X is a halogen atom or an inorganic radical: BX3 > AlX3 > FeX3 > GaX3 > SbX5 > SnX4 > AsX5 > ZnX2 > HgX2.
8.E.i Hard–Soft Acids–Bases
The facility with which an acid–base reaction takes place depends, of course, on the strengths of the acid and the base. But it also depends on quite another quality, called the hardness163 or softness of the acid or base.164 Hard and soft acids and bases have these characteristics:
Soft Bases. The donor atoms are of low electronegativity and high polarizability, and are easy to oxidize. They hold their valence electrons loosely.
Hard Bases. The donor atoms are of high electronegativity and low polarizability, and are hard to oxidize. They hold their valence electrons tightly.
Soft Acids. The acceptor atoms are large, have low positive charge, and contain unshared pairs of electrons (p or d) in their valence shells. They have high polarizability and low electronegativity.
Hard Acids. The acceptor atoms are small, have high positive charge, and do not contain unshared pairs in their valence shells. They have low polarizability and high electronegativity.
A qualitative listing of the hardness of some acids and bases is given in Table 8.3.165 The treatment has also been made quantitative,166 with the following operational definition:
![]()
In this equation, η, the absolute hardness, is half the difference between I, the ionization potential, and A, the electron affinity.167 The softness (σ), is the reciprocal of η. Values of η for some molecules and ions are given in Table 8.4.168 Note that the proton, which is involved in all Br![]() nsted acid–base reactions, is the hardest acid listed, with η = ∞ (it has no ionization potential). The above equation cannot be applied to anions, because electron affinities cannot be measured for them. Instead, the assumption is made that η for an anion X− is the same as that for the radical X•.169 Other methods are also needed to apply the treatment to polyatomic cations.169
nsted acid–base reactions, is the hardest acid listed, with η = ∞ (it has no ionization potential). The above equation cannot be applied to anions, because electron affinities cannot be measured for them. Instead, the assumption is made that η for an anion X− is the same as that for the radical X•.169 Other methods are also needed to apply the treatment to polyatomic cations.169
Table 8.3 Hard and Soft Acids and Basesa
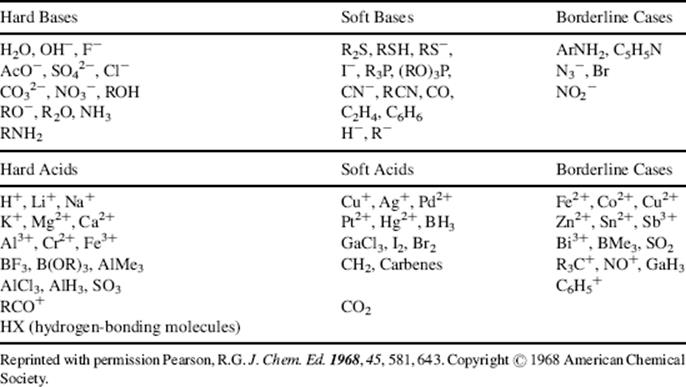
a. See Ref. 165.
Once acids and bases have been classified as hard or soft, a simple rule can be given: hard acids prefer to bond to hard bases, and soft acids prefer to bond to soft bases [the HSAB (hard–soft acid–base) principle].170 The rule has nothing to do with acid or base strength but merely says that the product A–B will have extra stability if both A and B are hard or if both are soft. Another rule is that a soft Lewis acid and a soft Lewis base tend to form a covalent bond, while a hard acid and a hard base tend to form ionic bonds.
One application of the first rule given above is found in complexes between alkenes or aromatic compounds and metal ions (see above). Alkenes and aromatic rings are soft bases and should prefer to complex with soft acids. Thus, Ag+, Pt2+, and Hg2+ complexes are common, but complexes of Na+, Mg2+, or Al3+ are rare. Chromium complexes are also common, but in such complexes the chromium is in a low or zero oxidation state (which softens it) or attached to other soft ligands. Another application is the reaction:
![]()
The HSAB principle predicts that the equilibrium should lie to the right, because the hard acid CH3CO+ should have a greater affinity for the hard base (RO−) than for the soft base (RS−). Indeed, thiol esters are easily cleaved by RO− or hydrolyzed by dilute base (−OH is also a hard base).171 Another application of the rule is discussed in Section 10.G.ii.172 The HSAB principles have been applied to analyze the reactivity of ketone and ester enolate anions,173 and in analyzing catalyst selectivity in synthesis.174