March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 11. Aromatic Substitution, Electrophilic
11.D. A Quantitative Treatment of Reactivity of the Electrophile: The Selectivity Relationship
Not all electrophiles are equally reactive. The nitronium ion is attacked not only by benzene but also by aromatic rings that contain a strongly deactivating group. On the other hand, diazonium ions couple only with rings containing a powerful activating group. Attempts have been made to correlate the influence of substituents with the reactivity of the group being attacked. The most obvious way to do this is with the Hammett equation (Sec. 8.G):
![]()
For aromatic substitution,91k0 is divided by 6 and, for meta substitution, k is divided by 2, so that comparisons are made for only one position (consequently, k/k0 for, say, the methyl group at a para position is identical to the partial rate factor pfMe). It was soon found that, while this approach worked fairly well for electron-withdrawing groups, it failed for those that are electron donating. However, if the equation is modified by the insertion of the Brown σ+values instead of the Hammett σ values (because a positive charge develops during the transition state), more satisfactory correlations can be made, even for electron-donating groups (see Table 9.4 for a list of σ+ values).92 Groups with a negative value of σp+ or σm+ are activating for that position; groups with a positive value are deactivating. The ρ values correspond to the susceptibility of the reaction to stabilization or destabilization by the Z group and to the reactivity of the electrophile. The ρ values vary not only with the electrophile, but also with conditions. A large negative value of ρ means an electrophile of relatively low reactivity. Of course, this approach is completely useless for ortho substitution, since the Hammett equation does not apply there.
A modification of the Hammett approach, suggested by Brown, called the selectivity relationship,93 is based on the principle that reactivity of a species varies inversely with selectivity. Table 11.3 shows how electrophiles can be arranged in order of selectivity as measured by two indexes: (1) their selectivity in attacking toluene rather than benzene, and (2) their selectivity between the meta and para positions in toluene.93 As the table shows, an electrophile more selective in one respect is also more selective in the other. In many cases, electrophiles known to be more stable (hence less reactive) than others show a higher selectivity, as would be expected. For example, the tert-butyl cation is more stable and more selective than the isopropyl (Sec. 5.A.ii), and Br2 is more selective than Br+. However, deviations from the relationship are known.94 Selectivity depends not only on the nature of the electrophile, but also on the temperature. As expected, it normally decreases with increasing temperature.
Table 11.3 Relative Rates and Product Distributions in Some Electrophilic Substitutions on Toluene and Benzenea
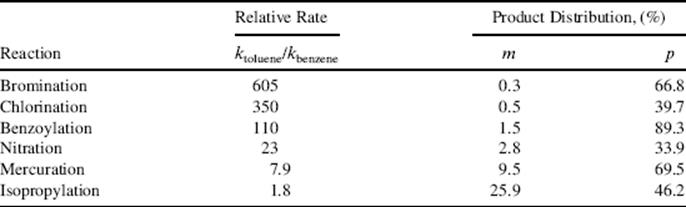
aSee Ref. 93.
Brown assumed that a good measurement of selectivity was the ratio of the para and meta partial rate factors in toluene. He defined the selectivity Sf of a reaction as:
![]()
That is, the more reactive the reactive species, the less preference it has for the para position compared to the meta. If we combine the Hammett–Brown σ+ρ relationship with the linearity between log Sf and log ![]() and between log Sf and log
and between log Sf and log ![]() , it is possible to derive the following expressions:
, it is possible to derive the following expressions:
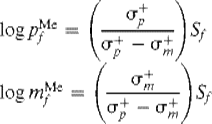
Sf is related to ρ by ![]()
The general validity of these equations is supported by a great deal of experimental data on aromatic substitution reactions of toluene. Examples of values for some reactions obtained from these equations are given in Table 11.4.95For other substituents, the treatment works well with groups that, like methyl, are not very polarizable. For more polarizable groups the correlations are sometimes satisfactory and sometimes not, probably because each electrophile in the transition state makes a different demand on the electrons of the substituent group.
Table 11.4 Values of ![]() ,
, ![]() , Sf, and ρ for Three Reactions of Toluenea
, Sf, and ρ for Three Reactions of Toluenea
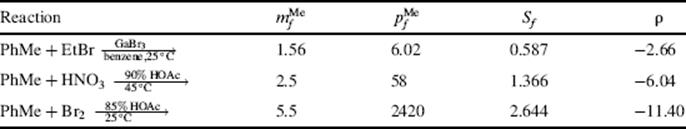
aSee Ref. 95.
Not only are there substrates for which the treatment is poor, but it also fails with very powerful electrophiles. This is the reason why it is necessary to postulate the encounter complex mentioned in Section 11.A.i. For example, relative rates of nitration of p-xylene, 1,2,4-trimethylbenzene, and 1,2,3,5-tetramethylbenzene were 1.0, 3.7, and 6.4,96 though the extra methyl groups should enhance the rates much more (p-xylene itself reacted 295 times faster than benzene). The explanation is that with powerful electrophiles the reaction rate is so rapid (reaction taking place at virtually every encounter97 between an electrophile and substrate molecule)98 that the presence of additional activating groups can no longer increase the rate.99
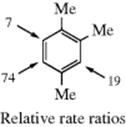
Given this behavior (little selectivity in distinguishing between different substrate molecules), the selectivity relationship would predict that positional selectivity should also be very small. However, it is not. For example, under conditions where nitration of p-xylene and 1,2,4-trimethylbenzene takes place at about equal rates, there was no corresponding lack of selectivity at positions within the latter.100 Although steric effects are about the same at both positions, >10 times as much 5-nitro product was formed as 6-nitro product. It is clear that the selectivity relationship has broken down and it becomes necessary to explain why such an extremely rapid reaction should occur with positional selectivity. The explanation offered is that the rate-determining step is formation of an encounter complex (12, Sec. 11.B.ii).101 Since the position of attachment is not determined in the rate-determining step, the 5:6 ratio is not related to the reaction rate. Essentially the same idea was suggested earlier102 and for the same reason (failure of the selectivity relationship in some cases), but the earlier explanation specifically pictured the complex as a π complex, and we have seen (Sec. 11.B.ii) that there is evidence against this.
One interesting proposal103 is that the encounter pair is a radical pair NO2√ ArH√+ formed by an electron transfer (SET), which would explain why the electrophile, once in the encounter complex, can acquire the selectivity that the free NO2+ lacked (it is not proposed that a radical pair is present in all aromatic substitutions; only in those that do not obey the selectivity relationship). The radical pair subsequently collapses to the arenium ion. There is evidence104 both for and against this proposal.105