March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 11. Aromatic Substitution, Electrophilic
11.C. Quantitative Treatments of Reactivity in the Substrate
Quantitative rate studies of aromatic substitutions are complicated by the fact that there are usually several hydrogen atoms that can leave, so that measurements of overall rate ratios do not give a complete picture as they do in nucleophilic substitutions, where it is easy to compare substrates that have only one possible leaving group in a molecule. What is needed is not, say, the overall rate ratio for acetylation of toluene versus that for benzene, but the rate ratio at each position. These can be calculated from the overall rates and a careful determination of the proportion of isomers formed, provided that the products are kinetically controlled, as is usually the case. The partial rate factormay be defined for a given group and a given reaction as the rate of substitution at a single position relative to a single position in benzene. For example, for acetylation of toluene the partial rate factors follow: for the ortho position ofMe = 4.5, for the meta mfMe = 4.8, and for the para pfMe = 749.85 This means that toluene is acetylated at the ortho position 4.5 times as fast as a single position in benzene, or 0.75 times as fast as the overall rate of acetylation of benzene. A partial rate factor >1 for a given position indicates that the group in question activates that position for the given reaction. Partial rate factors differ from one reaction to another and are even different, though less so, for the same reaction under different conditions.
Once the partial rate factors are known, the proportions of isomers to be obtained when two or more groups are present on a ring can be predicted, if the assumption is made that the effect of substituents is independent. For example, if the two methyl groups in m-xylene have the same effect as the methyl group in toluene, the theoretical partial rate factors at each position can be calculated by multiplying those from toluene, so they should be as indicated.
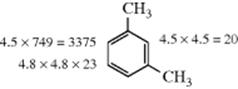
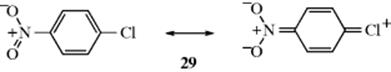
From this, it is possible to calculate the overall theoretical rate ratio for acetylation of m-xylene relative to benzene, since this is one-sixth the sum of the partial rate factors (in this case 1130), and the isomer distribution if the reaction is kinetically controlled. The overall rate ratio actually is 34786 and the calculated and observed isomer distributions are listed in Table 11.2.86 In this case, and in many others, agreement is fairly good, but many cases are known where the effects are not additive (as in Sec. 11.B.ii).87 For example, this treatment predicts that for 1,2,3-trimethylbenzene there should be 35% 5 substitution and 65% 4 substitution, but acetylation gave 79% 5 substitution and 21% of the 4 isomer. The treatment is thrown off by steric effects (e.g., those mentioned earlier, Sec. 11.B.iv), by products arising from ipso attack (Sec. 11.B.ii) and by resonance interaction between groups (e.g., 29), which must make the results deviate from simple additivity of the effects of the groups.
Table 11.2 Calculated and Experimental Isomer Distributions in the Acetylation of m-Xylenea
Isomer Distribution (%)
Position
Calculated
Observed
2
0.30
0
4
9.36
97.5
5
0.34
2.5
Reprinted with permission Marino G.; Brown, H.C. J. Am. Chem. Soc. 1959, 81, 5929. Copyright © 1959 American Chemical Society.
a. See Ref. 86.
Another approach that avoids the problem created by having competing leaving groups present in the same substrate is the use of substrates that contain only one leaving group. This is most easily accomplished by the use of a leaving group other than hydrogen. By this means, overall rate ratios can be measured for specific positions.88 Results obtained in this way89 give a reactivity order quite consistent with that for hydrogen as leaving group.
A quantitative scale of reactivity for aromatic substrates (fused, heterocyclic, and substituted rings) has been devised, based on the HSAB concept (Sec. 8.E).90 From MO theory, a quantity called activation hardness can be calculated for each position of an aromatic ring. The smaller the activation hardness, the faster the attachment at that position; hence the treatment predicts the most likely orientations for incoming groups.